Question: 4. These are half-reactions for denitrification with acetate substrate: Rd: 1/8 CH3COO + 3/8 H20 1/8 CO2 + 1/8 HCO3 + H+ +e Ra: 1/5
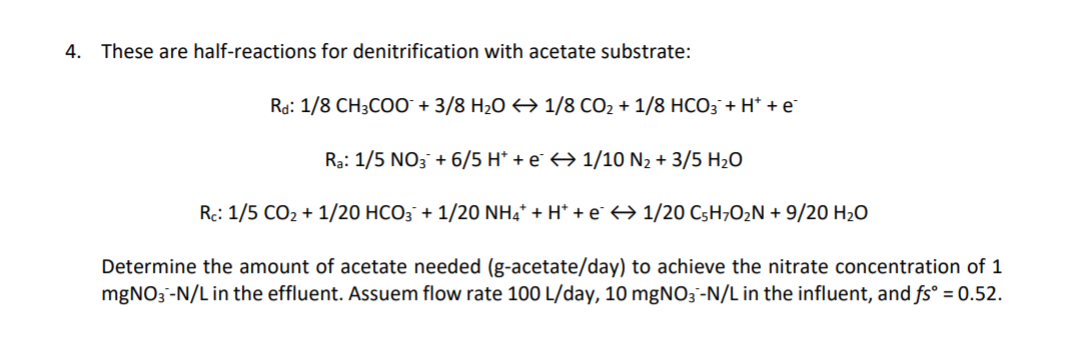
4. These are half-reactions for denitrification with acetate substrate: Rd: 1/8 CH3COO + 3/8 H20 1/8 CO2 + 1/8 HCO3 + H+ +e Ra: 1/5 NO3 + 6/5 H* + 1/10 N2 + 3/5 H20 Rc: 1/5 CO2 + 1/20 HCO3 + 1/20 NH4 + H+ + 1/20 CsH7O2N + 9/20 H2O Determine the amount of acetate needed (g-acetate/day) to achieve the nitrate concentration of 1 mgNO3-N/L in the effluent. Assuem flow rate 100 L/day, 10 mgNO3 --N/L in the influent, and fs = 0.52. 4. These are half-reactions for denitrification with acetate substrate: Rd: 1/8 CH3COO + 3/8 H20 1/8 CO2 + 1/8 HCO3 + H+ +e Ra: 1/5 NO3 + 6/5 H* + 1/10 N2 + 3/5 H20 Rc: 1/5 CO2 + 1/20 HCO3 + 1/20 NH4 + H+ + 1/20 CsH7O2N + 9/20 H2O Determine the amount of acetate needed (g-acetate/day) to achieve the nitrate concentration of 1 mgNO3-N/L in the effluent. Assuem flow rate 100 L/day, 10 mgNO3 --N/L in the influent, and fs = 0.52
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


