Question: 4) To determine the equilibrium just combine the initial and the change. J. Now we can write the equilibrium expression and plug in our equilibrium
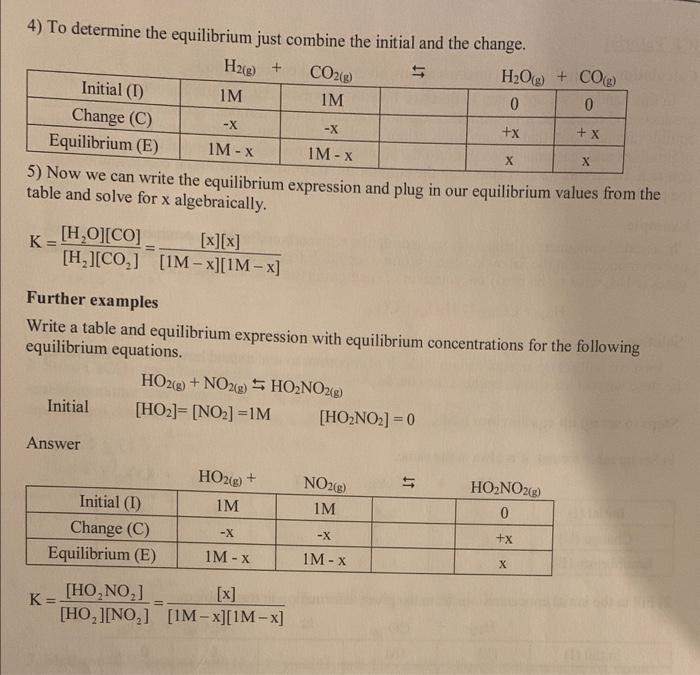
4) To determine the equilibrium just combine the initial and the change. J. Now we can write the equilibrium expression and plug in our equilibrium values from the table and solve for x algebraically. K=[H2][CO2][H2O][CO]=[1Mx][1Mx][x][x] Further examples Write a table and equilibrium expression with equilibrium concentrations for the following equilibrium equations. Initial HO2(g)+NO2(g)HO2NO2(g)[HO2]=[NO2]=1M[HO2NO2]=0 Answer K=[HO2][NO2][HO2NO2]=[1Mx][1Mx][x] 4) To determine the equilibrium just combine the initial and the change. J. Now we can write the equilibrium expression and plug in our equilibrium values from the table and solve for x algebraically. K=[H2][CO2][H2O][CO]=[1Mx][1Mx][x][x] Further examples Write a table and equilibrium expression with equilibrium concentrations for the following equilibrium equations. Initial HO2(g)+NO2(g)HO2NO2(g)[HO2]=[NO2]=1M[HO2NO2]=0 Answer K=[HO2][NO2][HO2NO2]=[1Mx][1Mx][x]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


