Question: 4. Using the ternary phase diagram (liquidus plot) given below answer the following questions: 15 a) How many binary eutectics are present in this system
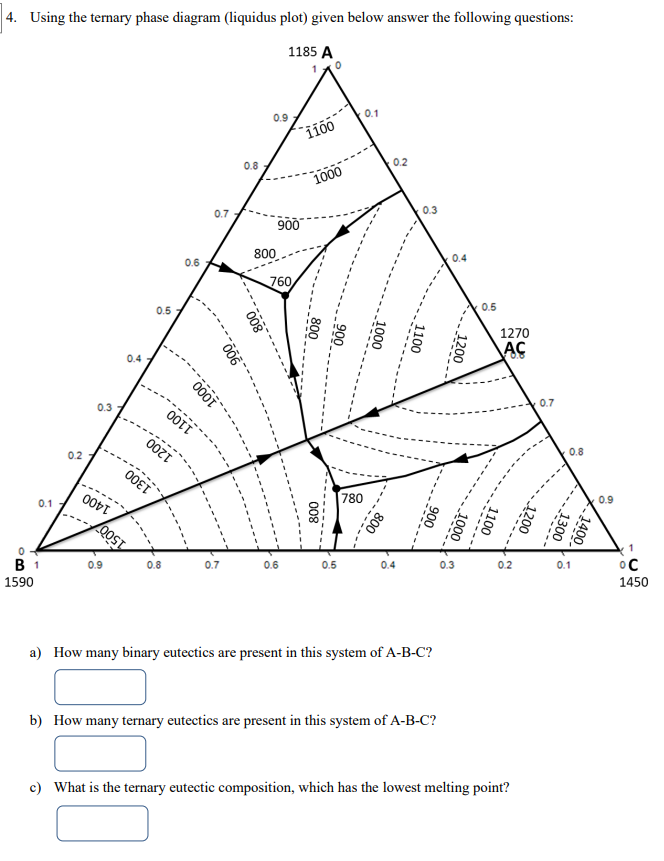
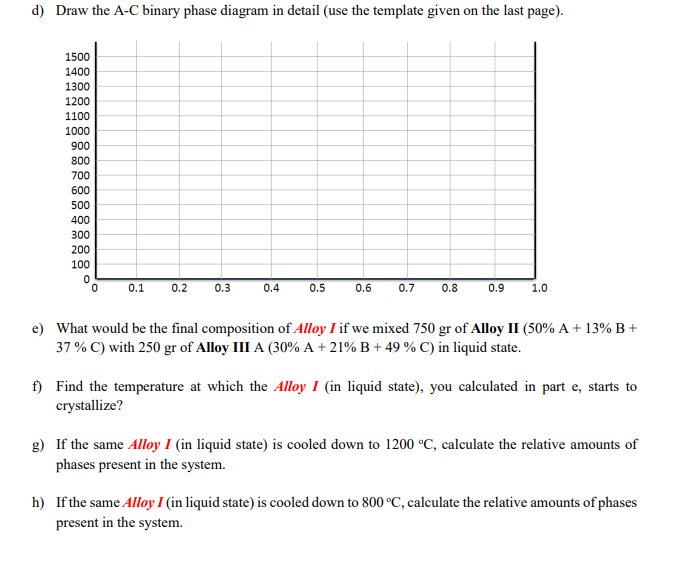
4. Using the ternary phase diagram (liquidus plot) given below answer the following questions: 15 a) How many binary eutectics are present in this system of A-B-C? b) How many ternary eutectics are present in this system of A-B-C? d) Draw the A-C binary phase diagram in detail (use the template given on the last page). e) What would be the final composition of Alloy I if we mixed 750 gr of Alloy II (50\% A + 13\% B + 37% C) with 250 gr of Alloy III A (30\% A +21%B+49% C) in liquid state. f) Find the temperature at which the Alloy I (in liquid state), you calculated in part e, starts to crystallize? g) If the same Alloy I (in liquid state) is cooled down to 1200C, calculate the relative amounts of phases present in the system. h) If the same Alloy I (in liquid state) is cooled down to 800C, calculate the relative amounts of phases present in the system
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


