Question: 4. Write a balanced chemical equation to represent the formation of one mole of ethane from its elements in their standard states. (1 mark) How
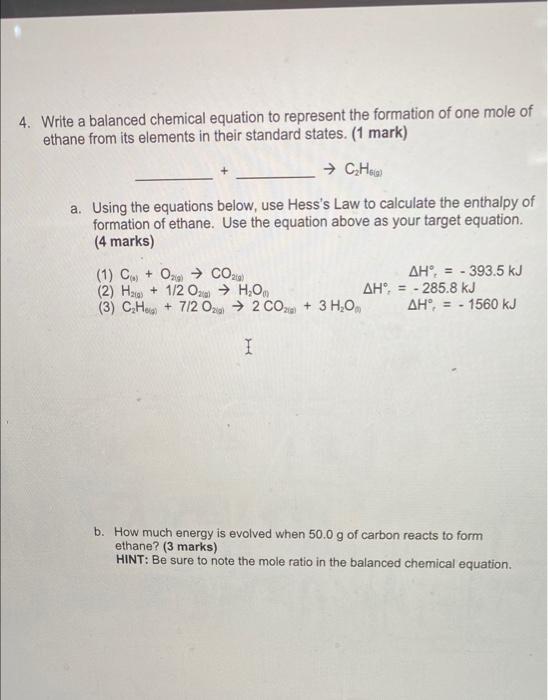
4. Write a balanced chemical equation to represent the formation of one mole of ethane from its elements in their standard states. (1 mark) How a. Using the equations below, use Hess's Law to calculate the enthalpy of formation of ethane. Use the equation above as your target equation. (4 marks) (1) C. + 0.29 CO24) AH, = - 393.5 kJ (2) Hag) + 1/2 O HOM AH, = - 285.8 kJ (3) CH + 7/2 02 2 CO2 + 3 H2O AH = - 1560 kJ 1 b. How much energy is evolved when 50.0 g of carbon reacts to form ethane? (3 marks) HINT: Be sure to note the mole ratio in the balanced chemical equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


