Question: 4. Write the equilibrium-constant expression for Kc for each of the following reactions: a) CO2(g)+H2(g)CO(g)+H2O(I) b) SnO2(s)+2CO(s)Sn(s)+2CO2(g) 5- For the Haber process, it is given
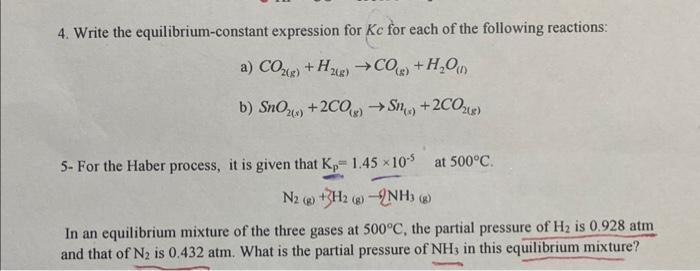
4. Write the equilibrium-constant expression for Kc for each of the following reactions: a) CO2(g)+H2(g)CO(g)+H2O(I) b) SnO2(s)+2CO(s)Sn(s)+2CO2(g) 5- For the Haber process, it is given that Kp=1.45105 at 500C. N2(g)+3H2(g)2NH3(b) In an equilibrium mixture of the three gases at 500C, the partial pressure of H2 is 0.928atm and that of N2 is 0.432atm. What is the partial pressure of NH3 in this equilibrium mixture
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


