Question: (40 pts) For two elementary, reversible reactions in series taking place in a batch reactor: ky k A B C K-1 k-2 CA E 1.6
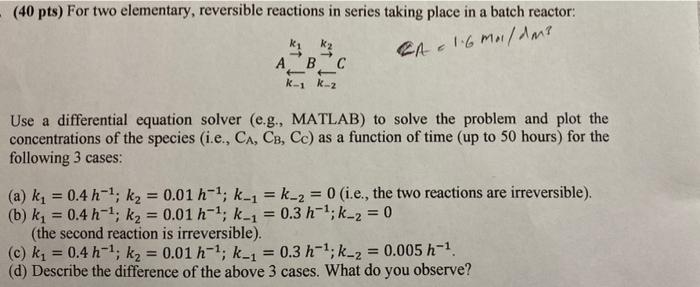
(40 pts) For two elementary, reversible reactions in series taking place in a batch reactor: ky k A B C K-1 k-2 CA E 1.6 malam? Use a differential equation solver (e.g., MATLAB) to solve the problem and plot the concentrations of the species (i.e., CA, CB, Cc) as a function of time (up to 50 hours) for the following 3 cases: = = (a) k, = 0.4 h-1; k, = 0.01 h-1; k_1 = k-2 = 0 (i.e., the two reactions are irreversible). (b) k, = 0.4 h-1, kg = 0.01 h-1, k-1 = 0.3 h-1; k_2 = 0 (the second reaction is irreversible). (c) k, = 0.4 h-1; ky = 0.01 h-1, k-1 = 0.3 h-1;k_2 = 0.005 h-1 (d) Describe the difference of the above 3 cases. What do you observe? = =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


