Question: please solve it in polymath ODE ONLY NO MATLAB 2. (30 pts) Consider two elementary, non-reversible reactions in parallel carried out in a batch reactor:
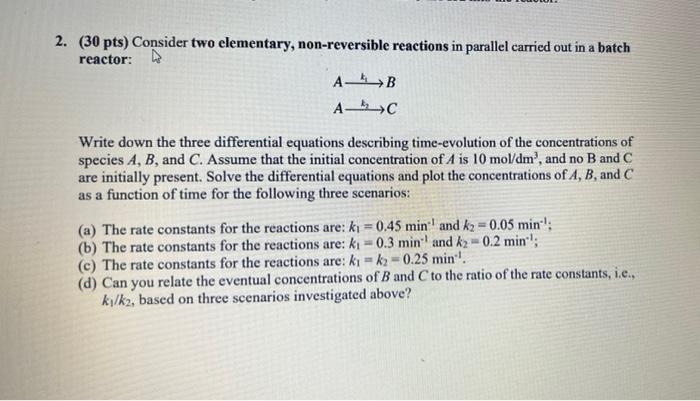
2. (30 pts) Consider two elementary, non-reversible reactions in parallel carried out in a batch reactor: AB AC Write down the three differential equations describing time-evolution of the concentrations of species A, B, and C. Assume that the initial concentration of A is 10 mol/dm', and no B and C are initially present. Solve the differential equations and plot the concentrations of A, B, and C as a function of time for the following three scenarios: (a) The rate constants for the reactions are: ki = 0.45 min. and k2 = 0.05 min (b) The rate constants for the reactions are: ki = 0.3 min and k2 -0.2 min; (c) The rate constants for the reactions are: kk2-0.25 min''. (d) Can you relate the eventual concentrations of B and C to the ratio of the rate constants, i.e., ki/k2, based on three scenarios investigated above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


