Question: 400 L 300 Liquidus Solidus a + L Temperature (C) 200 Liquidus L+B 97.5 Solidus B 19 183 61.9 Solvus Solvus 100 a+B | Sn
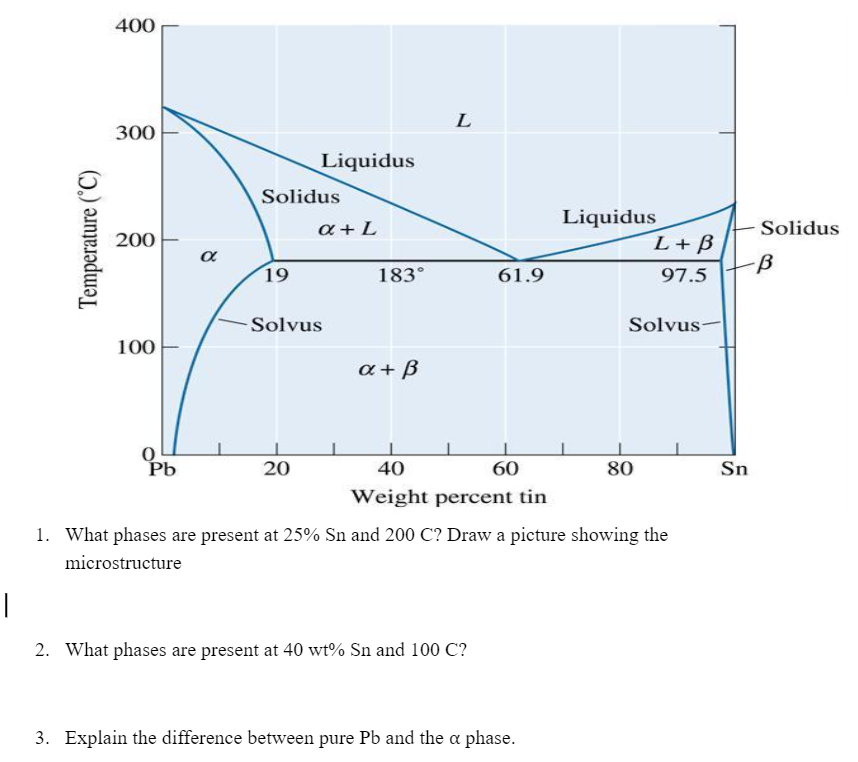
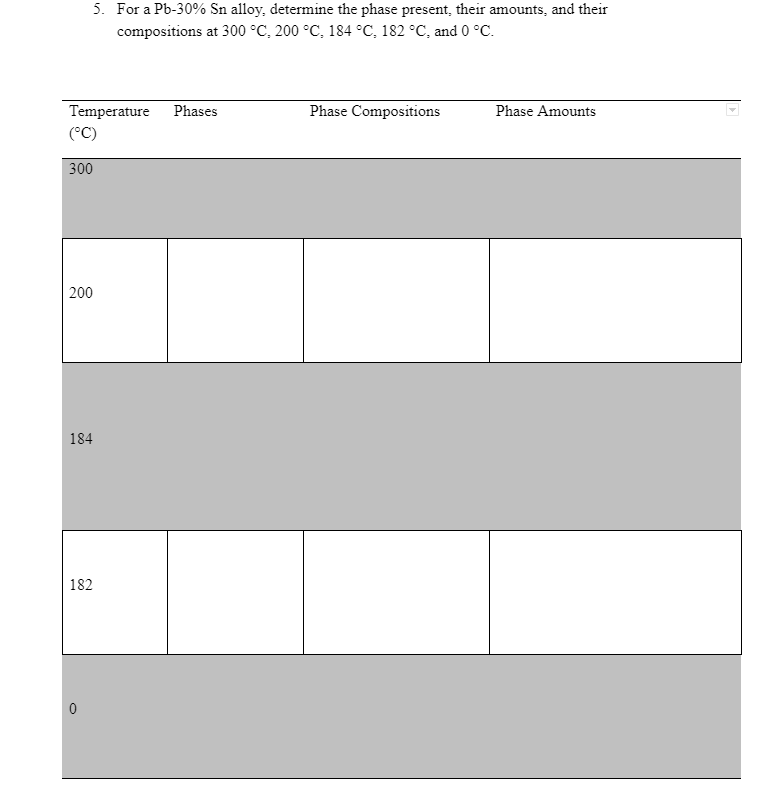
400 L 300 Liquidus Solidus a + L Temperature (C) 200 Liquidus L+B 97.5 Solidus B 19 183 61.9 Solvus Solvus 100 a+B | Sn 0 Pb 20 40 60 80 Weight percent tin 1. What phases are present at 25% Sn and 200 C? Draw a picture showing the microstructure | 2. What phases are present at 40 wt% Sn and 100 C? 3. Explain the difference between pure Pb and the a phase. 4. Identify all compositions on the lead-tin phase diagram for which melting occurs at a single temperature. What are those temperatures? (Don't just look for intersections of lines on the phase diagram. Draw lines showing the path the material takes during cooling to see where melting occurs at a single temperature.) 5. For a Pb-30% Sn alloy, determine the phase present their amounts, and their compositions at 300 C. 200 C. 184C, 182 C, and 0 C. Phase Compositions Phase Amounts Temperature Phases (C) 300 200 184 182 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


