Question: Given: Lead (Pb) - Tin (Sn) Phase Diagram Composition (at% Sn) 0 20 40 60 80 100 1 327C * (A) 1 600 300 Liquid
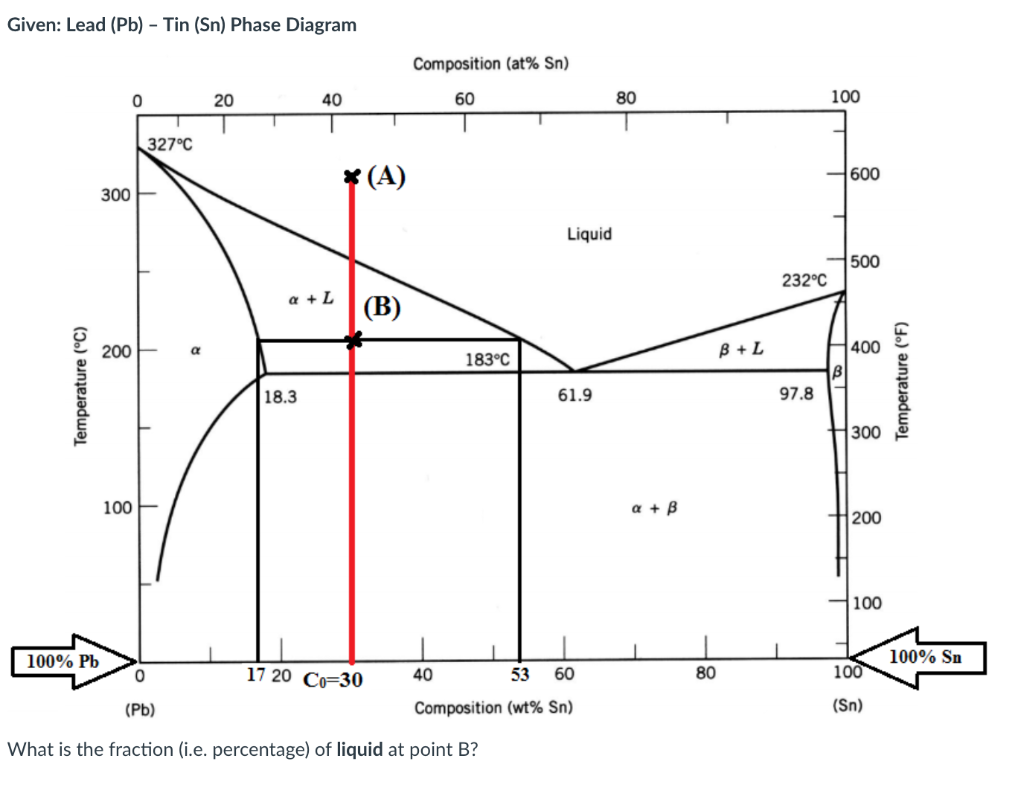
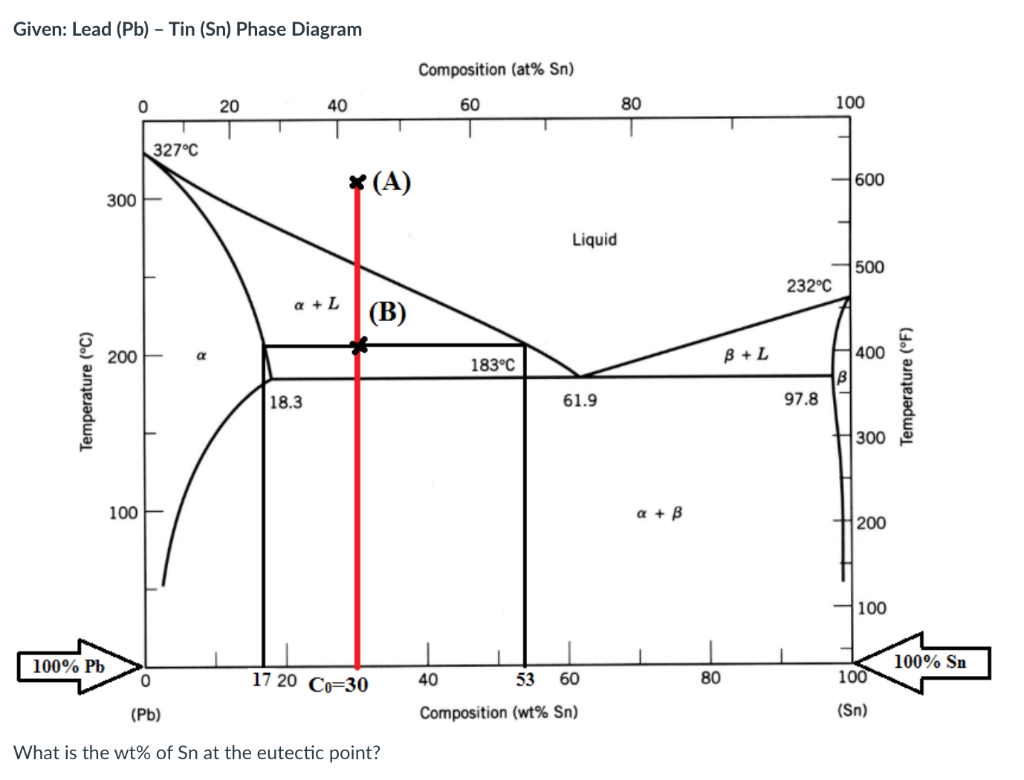
Given: Lead (Pb) - Tin (Sn) Phase Diagram Composition (at% Sn) 0 20 40 60 80 100 1 327C * (A) 1 600 300 Liquid 500 232C Q + L (B) 200 B + L 183C 400 B Temperature (C) Temperature (F) 18.3 61.9 97.8 300 100 a + B 200 100 100% Pb 100% Sn 0 17 20 Co=30 40 53 60 80 100 (Pb) Composition (wt% Sn) (Sn) What is the fraction (i.e. percentage) of liquid at point B? Given: Lead (Pb) - Tin (Sn) Phase Diagram Composition (at% Sn) 0 20 40 60 80 100 327C (A) 600 300 Liquid 500 232C a + L (B) 200 a 183C B + L 400 B Temperature (C) Temperature (F) 18.3 61.9 97.8 300 100 a + B 200 100 100% Pb 100% Sn 0 17 20 Co=30 40 53 60 80 100 (Pb) Composition (wt% Sn) (Sn) What is the wt% of Sn at the eutectic point
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


