Question: 41) Given the following data: (1) N2(g) + O2(g) 2NO(g) Kc1 = 4.3 x 1025 (2) 2NO(g) + O2(g) 2NO2(g) Kc2= 6.4 x 10
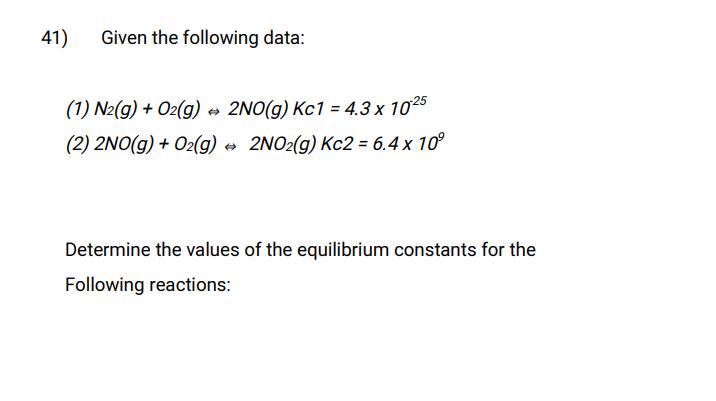
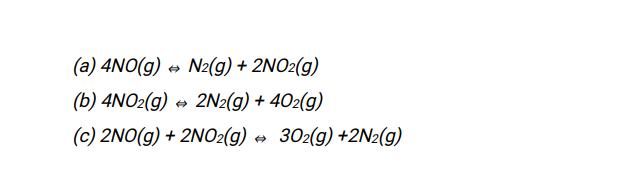
41) Given the following data: (1) N2(g) + O2(g) 2NO(g) Kc1 = 4.3 x 1025 (2) 2NO(g) + O2(g) 2NO2(g) Kc2= 6.4 x 10 Determine the values of the equilibrium constants for the Following reactions: (a) 4NO(g) N2(g) + 2NO2(g) (b) 4NO2(g) 2N2(g) + 402(g) (c) 2NO(g) + 2NO(g) 302(g) +2N(g)
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


