Question: 4A9 (5) + 2H SC ) + How much heat is released when 6.38 grams of Ag(s) (m.m = 107.9 g/mol) reacts by the equation
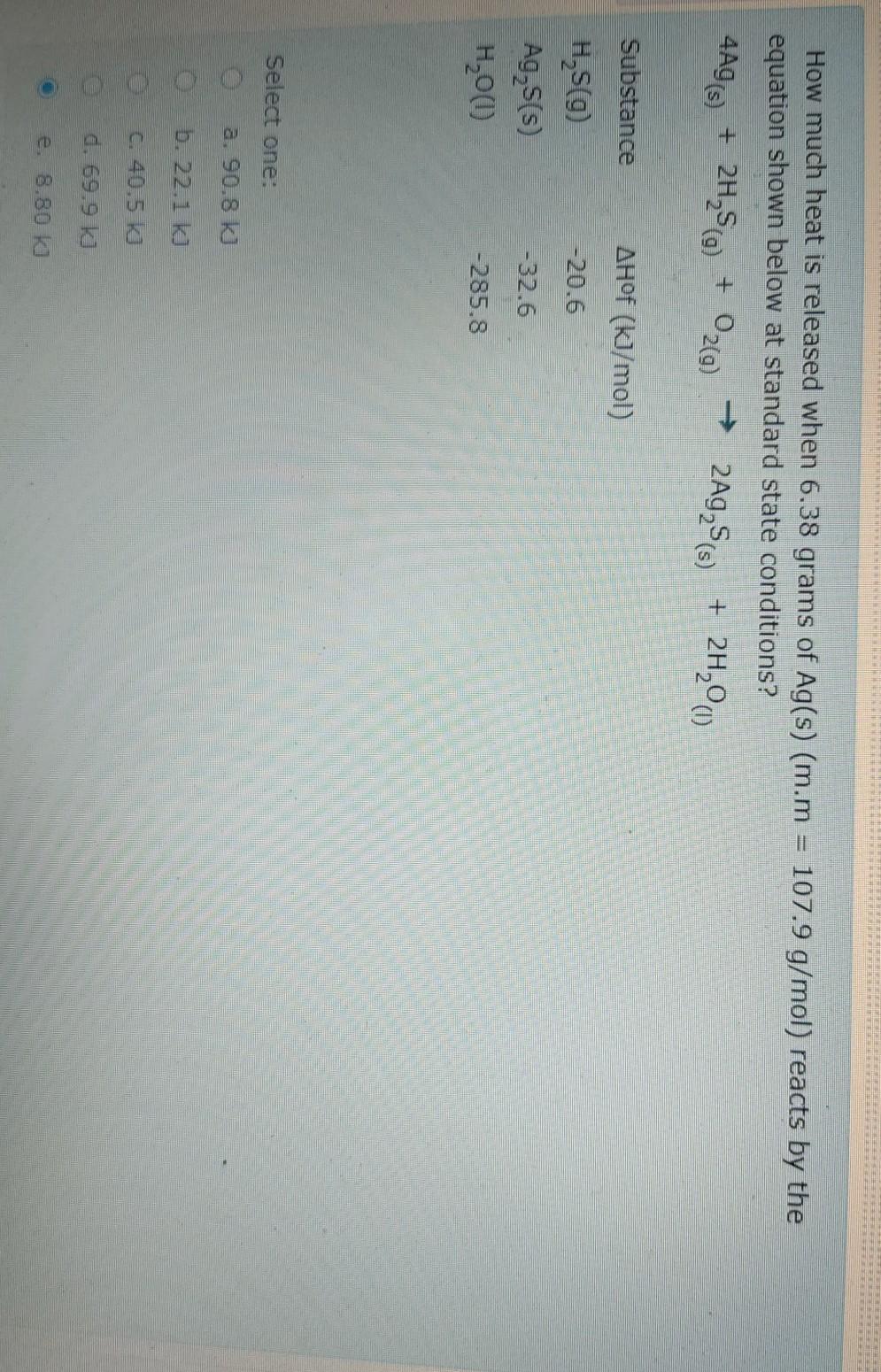
4A9 (5) + 2H SC ) + How much heat is released when 6.38 grams of Ag(s) (m.m = 107.9 g/mol) reacts by the equation shown below at standard state conditions? 2Ag,$s) + 2H00 020) Substance AHOF (kJ/mol) -20.6 H, (9 Ag, 5(s) H2000) -32.6 -285.8 Select one: a. 90.8 kJ b.22.1 k) C. 40.5 kJ d. 69.9 kJ e. 8.80 kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


