Question: 4(e) Consider the reversible reaction between nitrogen and oxygen at high temperatures: 2H2(g)+CO(g)CH3OH(g)H=90.2kJ (i) What will happen to the CO concentration at equilibrium if more
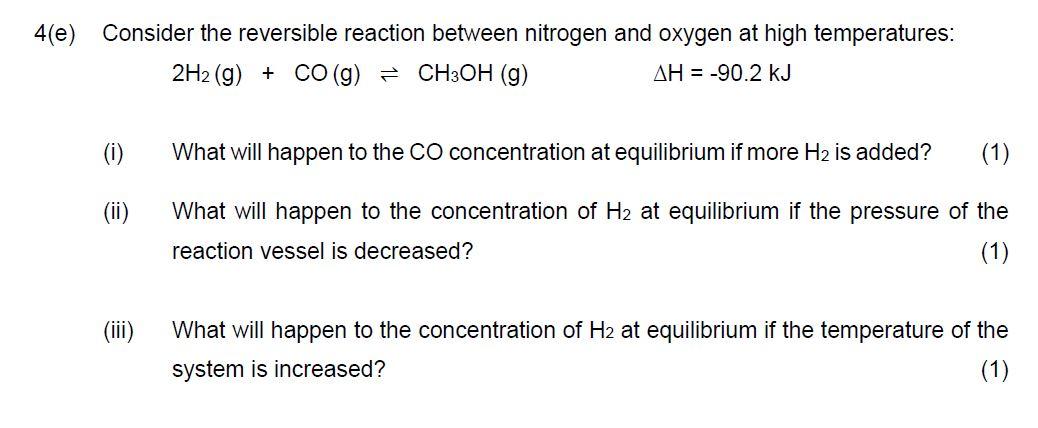
4(e) Consider the reversible reaction between nitrogen and oxygen at high temperatures: 2H2(g)+CO(g)CH3OH(g)H=90.2kJ (i) What will happen to the CO concentration at equilibrium if more H2 is added? (ii) What will happen to the concentration of H2 at equilibrium if the pressure of the reaction vessel is decreased? (iii) What will happen to the concentration of H2 at equilibrium if the temperature of the system is increased
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


