Question: Q 12.4 a Consider a reversible reaction with a 4 G value of -20 kJ. mol-'. In which direction will this reaction occur at the
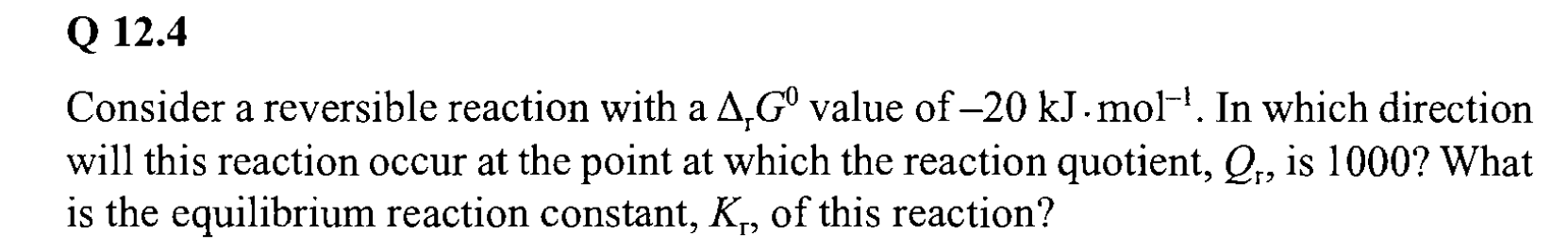
Q 12.4 a Consider a reversible reaction with a 4 G value of -20 kJ. mol-'. In which direction will this reaction occur at the point at which the reaction quotient, , is 1000? What is the equilibrium reaction constant, K,, of this reaction? T
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


