Question: ( 5 0 points ) A 2 0 L batch of hard water is treated to remove with a strong acid cation ion - exchange
points A L batch of hard water is treated to remove with a strong acid cation ionexchange resins
made of polystyrene with DVB crosslinking The hard water initially contains and
The capacity of the resins is and the resins are initially charged with a What
is the volume of the resins that is needed to remove in the solution? b After the ion
exchange process is completed, regeneration of the resin is achieved by transferring the resins into a
L highly concentrated sodium chloride solution. Calculate the final concentrations of and
in the solution, and the concentrations of and NaR in the resin.
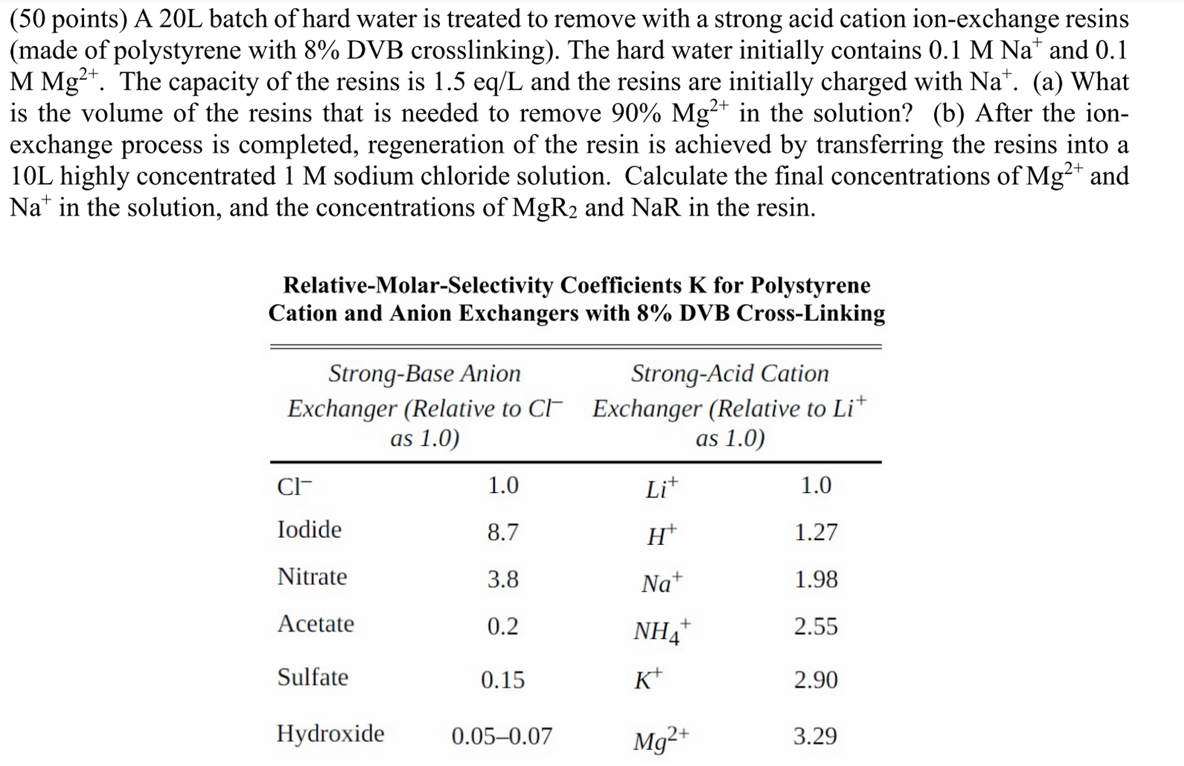
RelativeMolarSelectivity Coefficients for Polystyrene
Cation and Anion Exchangers with DVB CrossLinking
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


