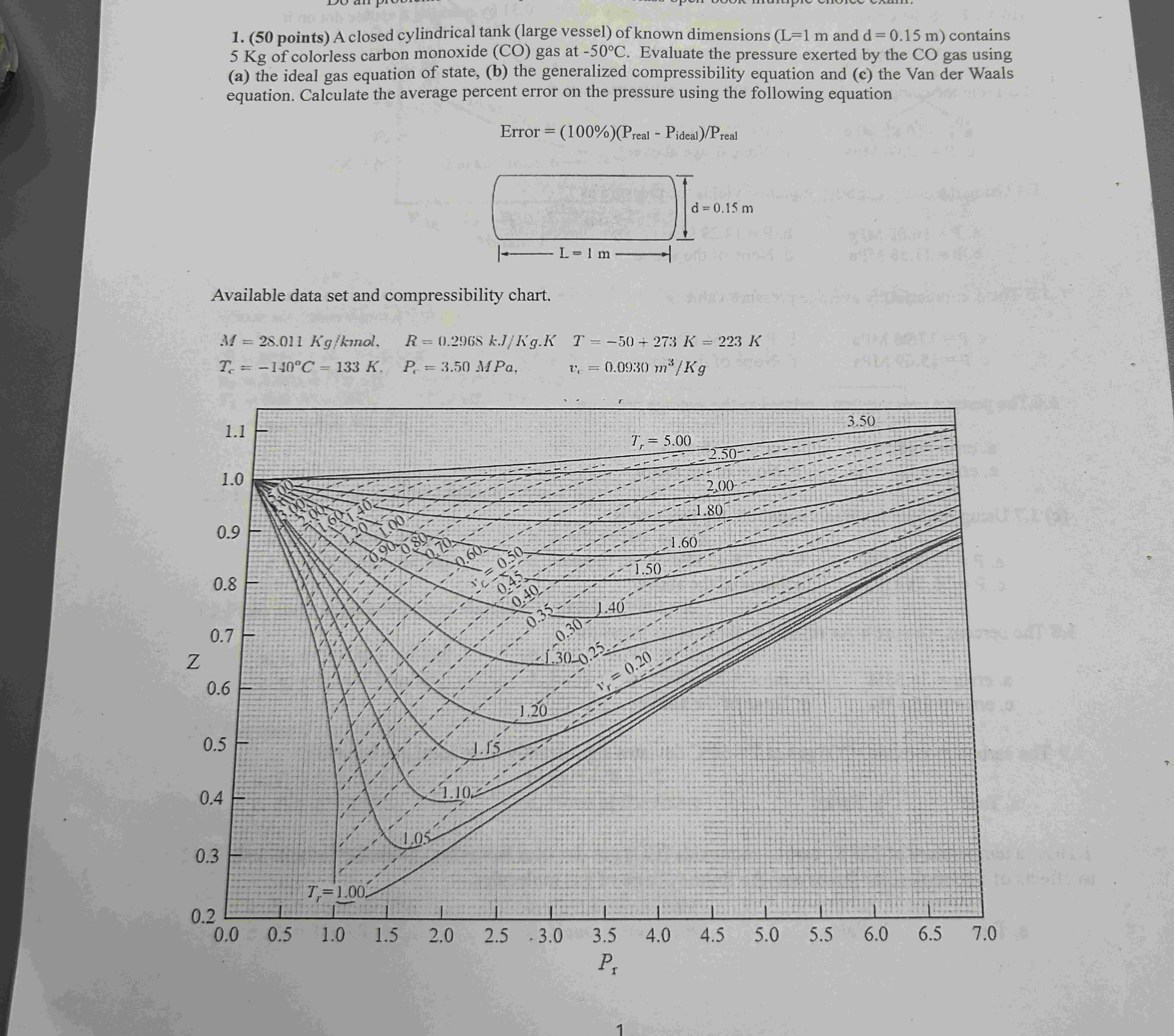
Question: 5 0 pointsL = 1 m and d = 0 . 1 5 m - 5 0 deg C . Evaluate the pressure exerted
pointsLm and dm deg C Evaluate the pressure exerted by the CO gas using a the ideal gas equation of state, b the generalized compressibility equation and c the Van der Waals equation. Calculate the average percent error on the pressure using the following equation
Error Preal Pideal Preal
Available data set and compressibility chart.
MKgkmolRkJKgKTKK
Tcdeg CKPrMPa,vcmKg
a The ideal equation of state EOS yields the pressure as
a PMPa
b PkPa
c PMPa
d None of the above
b From the generalized compressibility chart, verify that vr by marking a visible dot on it
a Done correctly
b Done incorrectly
From the compressibility chart, the corresponding pressure is
a PMPa
b PkPa
c PMPa
d None of the above
Using the compressibility equation yields the pressure as
a PMPa
b PkPa
c PMPa
d None of the above
The compressibility average pressure value is
a PMPa
b PkPa
c PMPa
d None of the above
The percent pressure error related to the average pressure
a error
b error
c error
d None of the above
c Using the Van der Waals equation, the pressure is
a PMPa
b PkPa
c PMPa
d None of the above
The percent pressure error related to the average pressure
a error
b error
c error
d None of the above
The carbon monoxide CO gas at Tdeg C deviates significantly from ideal gas behavior.
a True
b False
At a temperature of K carbon monoxide CO gas deviates from ideal gas behavior primarily due
to effects of intermolecular forces and the finite volume of gas molecules.
a True
b False
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


