Question: 5. ( 0.5pts) Given the zero-order gas phase reaction at 500K:N2O42NO2, with a rate constant of 1.00104Ms1, and an initial concentration of 0.250M, a) Write
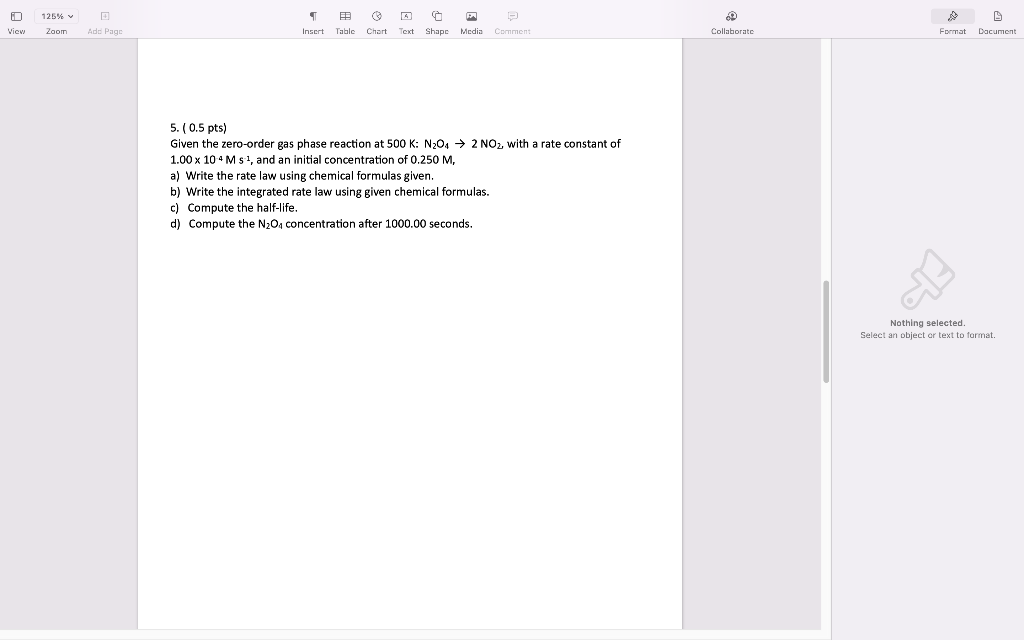
5. ( 0.5pts) Given the zero-order gas phase reaction at 500K:N2O42NO2, with a rate constant of 1.00104Ms1, and an initial concentration of 0.250M, a) Write the rate law using chemical formulas given. b) Write the integrated rate law using given chemical formulas. c) Compute the half-life. d) Compute the N2O4 concentration after 1000.00 seconds
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


