Question: 5. (10 Points) The figure below shows two identical copper blocks of mass m=1.5 kg: block L at temperature 60C and block R at temperature
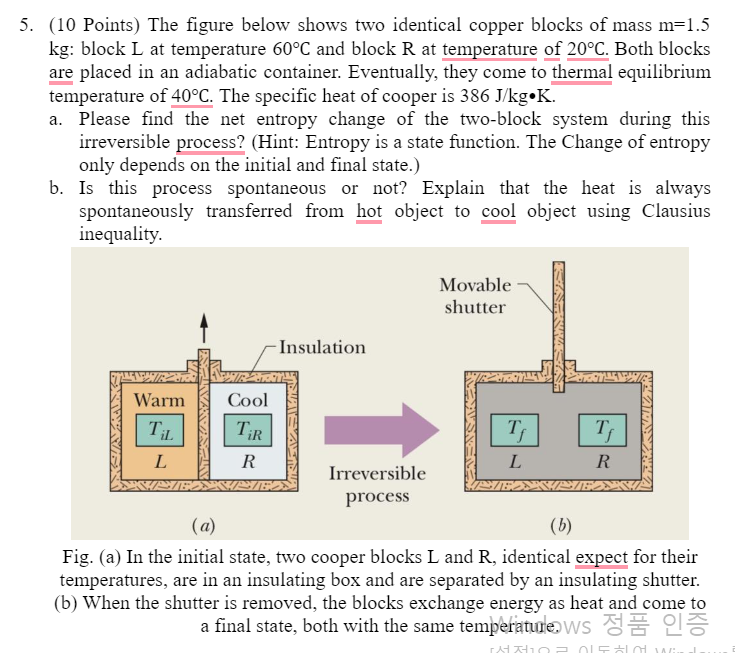
5. (10 Points) The figure below shows two identical copper blocks of mass m=1.5 kg: block L at temperature 60C and block R at temperature of 20C. Both blocks are placed in an adiabatic container. Eventually, they come to thermal equilibrium temperature of 40C. The specific heat of cooper is 386J/kgK. a. Please find the net entropy change of the two-block system during this irreversible process? (Hint: Entropy is a state function. The Change of entropy only depends on the initial and final state.) b. Is this process spontaneous or not? Explain that the heat is always spontaneously transferred from hot object to cool object using Clausius inequality. Fig. (a) In the initial state, two cooper blocks L and R, identical expect for their temperatures, are in an insulating box and are separated by an insulating shutter. (b) When the shutter is removed, the blocks exchange energy as heat and come to a final state, both with the same temperature.ws
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


