Question: 5. (18%) A chemical solution contains N type A molecules and A/ type B molecules. Type A and type B molecules undergo an irreversible reaction
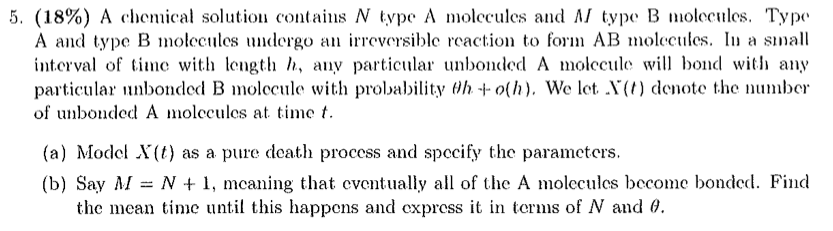
5. (18%) A chemical solution contains N type A molecules and A/ type B molecules. Type A and type B molecules undergo an irreversible reaction to form AB molecules. In a small interval of time with length h, any particular unbonded A molecule will bond with any particular unbonded B molecule with probability th + o(h). We let. X(t) denote the number of unbonded A molecules at time f. (a) Model X(t) as a pure death process and specify the parameters. (b) Say M = N + 1, meaning that eventually all of the A molecules become bonded. Find the mean time until this happens and express it in terms of N and 9
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


