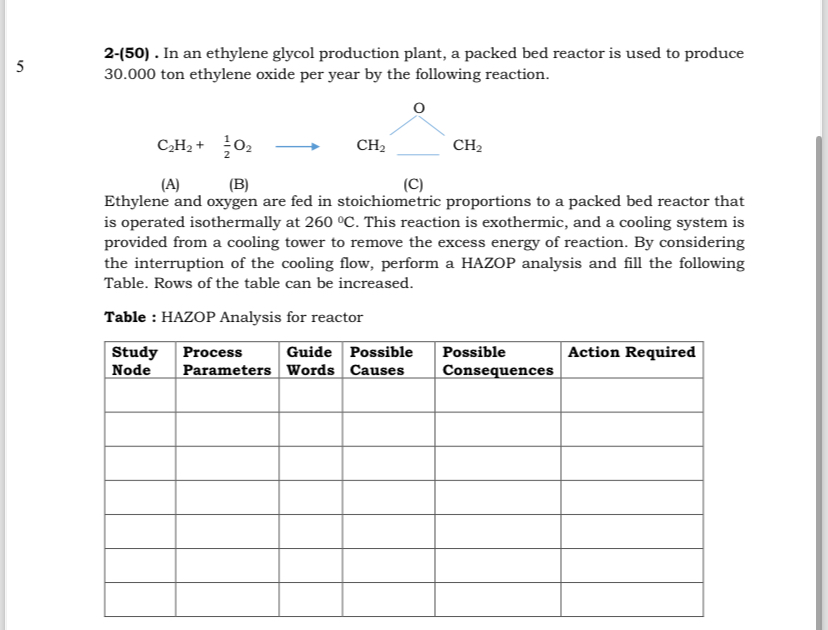
Question: 5 2 - ( 5 0 ) . In an ethylene glycol production plant, a packed bed reactor is used to produce 3 0 .
In an ethylene glycol production plant, a packed bed reactor is used to produce ton ethylene oxide per year by the following reaction.
A
B
C
Ethylene and oxygen are fed in stoichiometric proportions to a packed bed reactor that is operated isothermally at This reaction is exothermic, and a cooling system is provided from a cooling tower to remove the excess energy of reaction. By considering the interruption of the cooling flow, perform a HAZOP analysis and fill the following Table. Rows of the table can be increased.
Table : HAZOP Analysis for reactor
tabletableStudyNodetableProcessParameterstableGuideWordstablePossibleCausestablePossibleConsequencesAction Required
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


