Question: 5. (30%) For a reaction : A(g)+2B(g)C(g) The experimental data at 300K are shown in the table. Answer questions: (a) Calculate the order with respect
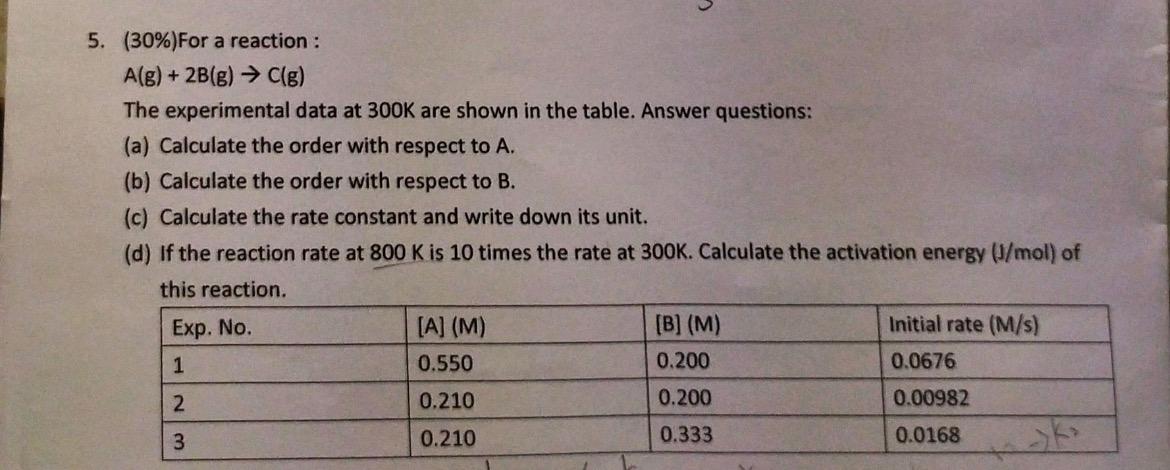
5. (30%) For a reaction : A(g)+2B(g)C(g) The experimental data at 300K are shown in the table. Answer questions: (a) Calculate the order with respect to A. (b) Calculate the order with respect to B. (c) Calculate the rate constant and write down its unit. (d) If the reaction rate at 800K is 10 times the rate at 300K. Calculate the activation energy (J/mol) of this reaction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


