Question: 5 A . A pharmaceutical drug A was quantified by pulse voltammetry; it had an E 1 2 value 5 A . A pharmaceutical drug
A A pharmaceutical drug A was quantified by pulse voltammetry; it had an valueA A pharmaceutical drug A was quantified by pulse voltammetry; it had an value
of vs SCE in A sample containing A gave a wave
amplitude of When of in were added to the
sample, the wave amplitude increased to
Compute the molarity of in the unknown.
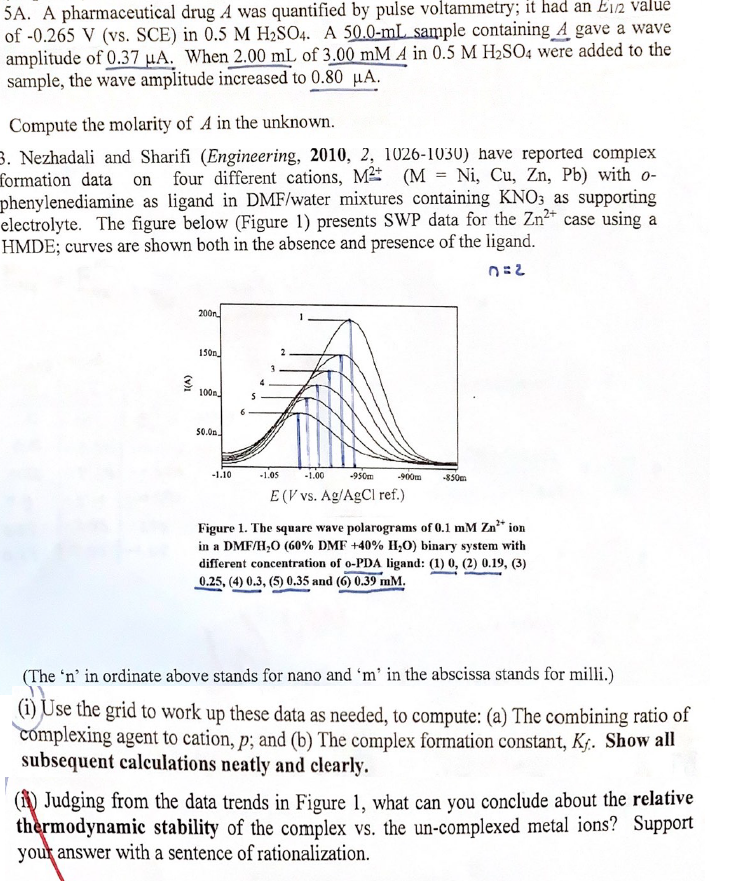
Nezhadali and Sharifi Engineering have reported complex
formation data on four different cations, with
phenylenediamine as ligand in DMFwater mixtures containing as supporting
electrolyte. The figure below Figure presents SWP data for the case using a
HMDE; curves are shown both in the absence and presence of the ligand.
Figure The square wave polarograms of ion
in a binary system with
different concentration of PDA ligand:
and
The in ordinate above stands for nano and in the abscissa stands for milli.
i
i Use the grid to work up these data as needed, to compute: a The combining ratio of
complexing agent to cation, ; and b The complex formation constant, Show all
subsequent calculations neatly and clearly.
ii Judging from the data trends in Figure what can you conclude about the relative
thermodynamic stability of the complex vs the uncomplexed metal ions? Support
you answer with a sentence of rationalization.
of vs SCE in A sample containing A gave a wave
amplitude of When of mMA in were added to the
sample, the wave amplitude increased to
B Compute the molarity of in the unknown.
Nezhadali and Sharifi Engineering have reported complex
formation data on four different cations, with
phenylenediamine as ligand in DMFwater mixtures containing as supporting
electrolyte. The figure below Figure presents SWP data for the case using a
HMDE; curves are shown both in the absence and presence of the ligand.
Figure The square wave polarograms of ion
in a binary system with
different concentration of PDA ligand:
and
The in ordinate above stands for nano and in the abscissa stands for milli.
i Use the grid to work up these data as needed, to compute: a The combining ratio of
complexing agent to cation, ; and b The complex formation constant, Show all
subsequent calculations neatly and clearly.
ii Judging from the data trends in Figure what can you conclude about the relative
thermodynamic stability of the complex vs the uncomplexed metal ions? Support
yout answer with a sentence of rationalization.
iii I read from the above paper under the Experimental Section that the SWP frequency
was and the potential scan rate was What would have been the staircase
shift used in these experiments?
Answer all questions together, It is a full question
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


