Question: 5. (a) Dislocations are called defects, but they also have a positive effect on the material. Explain these effect (b) Atomic Radius, crystal structure, electronegativity,
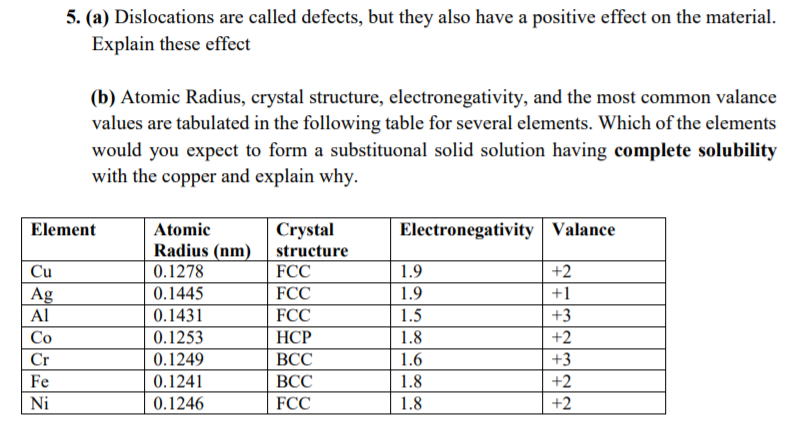
5. (a) Dislocations are called defects, but they also have a positive effect on the material. Explain these effect (b) Atomic Radius, crystal structure, electronegativity, and the most common valance values are tabulated in the following table for several elements. Which of the elements would you expect to form a substituonal solid solution having complete solubility with the copper and explain why. Element Electronegativity Valance Cu Ag Cr Fe Ni Atomic Crystal Radius (nm) structure 0.1278 FCC 0.1445 FCC 0.1431 FCC 0.1253 HCP 0.1249 0.1241 BCC 0.1246 FCC 1.9 1.9 1.5 1.8 1.6 1.8 1.8 +2 +1 +3 +2 +3 +2 +2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


