Question: (5) As we learned in our class, anharmonic oscillator represents diatomic molecules better than the 1D-HO. According to this model, frequency (in cm1) of IR
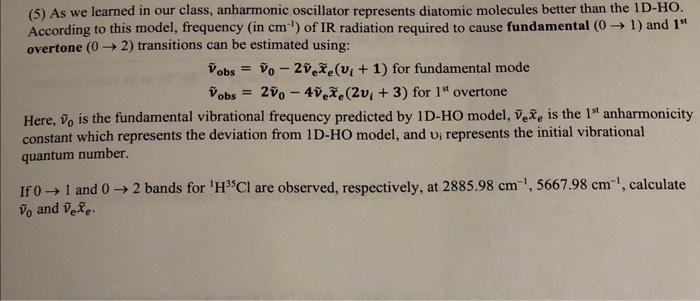
(5) As we learned in our class, anharmonic oscillator represents diatomic molecules better than the 1D-HO. According to this model, frequency (in cm1) of IR radiation required to cause fundamental (01) and 1ti overtone (02) transitions can be estimated using: v~obs=v~02v~ex~e(vi+1)forfundamentalmodev~obs=2v~04v~ex~e(2vi+3)for1stovertone Here, v~0 is the fundamental vibrational frequency predicted by 1D-HO model, v~ex~e is the 1st anharmonicity constant which represents the deviation from 1D-HO model, and vi represents the initial vibrational quantum number. If 01 and 02 bands for 1H35Cl are observed, respectively, at 2885.98cm1,5667.98cm1, calculate v~0 and v~ex~e
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


