Question: In a monoclinic crystal with a = 5 , b = 4 , c = 3 , and = 95, there is a metal
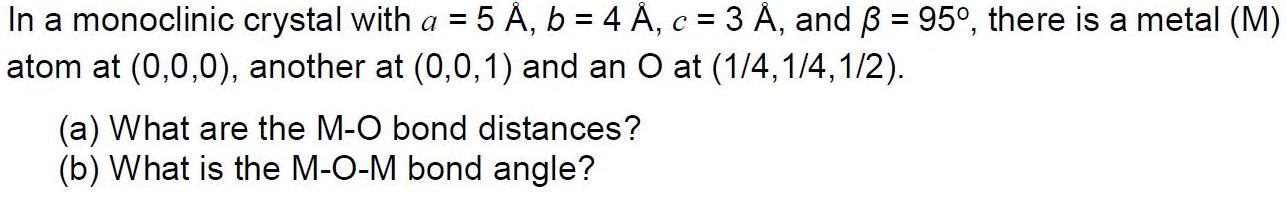
In a monoclinic crystal with a = 5 , b = 4 , c = 3 , and = 95, there is a metal (M) atom at (0,0,0), another at (0,0,1) and an O at (1/4,1/4,1/2). (a) What are the M-O bond distances? (b) What is the M-O-M bond angle?
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
A typical monoclinic structure is shown below Metal atom M ... View full answer

Get step-by-step solutions from verified subject matter experts


