Question: 5. Consider a solution produced by adding enough A to pure water to see a precipitate. a. Is the dissolution reaction exothermic or endothermic? b.
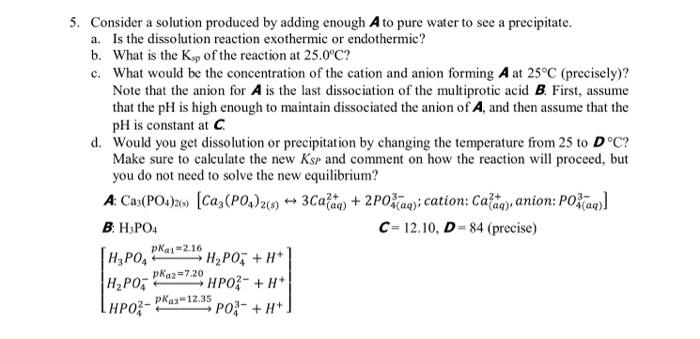
5. Consider a solution produced by adding enough A to pure water to see a precipitate. a. Is the dissolution reaction exothermic or endothermic? b. What is the Ksp of the reaction at 25.0C ? c. What would be the concentration of the cation and anion forming A at 25C (precisely)? Note that the anion for A is the last dissociation of the multiprotic acid B. First, assume that the pH is high enough to maintain dissociated the anion of A, and then assume that the pH is constant at C. d. Would you get dissolution or precipitation by changing the temperature from 25 to DC ? Make sure to calculate the new KSP and comment on how the reaction will proceed, but you do not need to solve the new equilibrium? A:Ca3(PO4)2(s)[Ca3(PO4)2(s)3Ca(aq)2++2PO4(aq)3; cation: Ca(aq)2+, anion: PO4(aq)3] B: H3PO4 C=12.10,D=84 (precise) H3PO4pKa1=2.16H2PO4+H+H2PO4pKa2=7.20HPO42+H+HPO42pa2=12.35PO43+H+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


