Question: 5. Consider the reaction: TiO2 (s) + 2C (graphite) + 2Cl2 (g) = 2CO (g) + TiCl4 (1) for which AH (298K) = -80.01
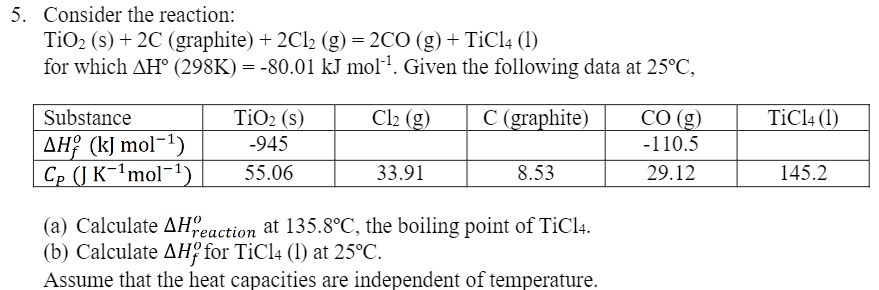
5. Consider the reaction: TiO2 (s) + 2C (graphite) + 2Cl2 (g) = 2CO (g) + TiCl4 (1) for which AH (298K) = -80.01 kJ mol. Given the following data at 25C, Substance TiO2 (s) Cl2 (g) C (graphite) CO (g) TiCl4 (1) AH (kJ mol-1) -945 -110.5 Cp (J Kmol-) 55.06 33.91 8.53 29.12 145.2 (a) Calculate AHreaction at 135.8C, the boiling point of TiCl4. (b) Calculate AH for TiCl4 (1) at 25C. Assume that the heat capacities are independent of temperature.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


