Question: 5. Evaluate the difference between change in energy at 0K in the absence of zero point vibration and both change in enthalpy and in free
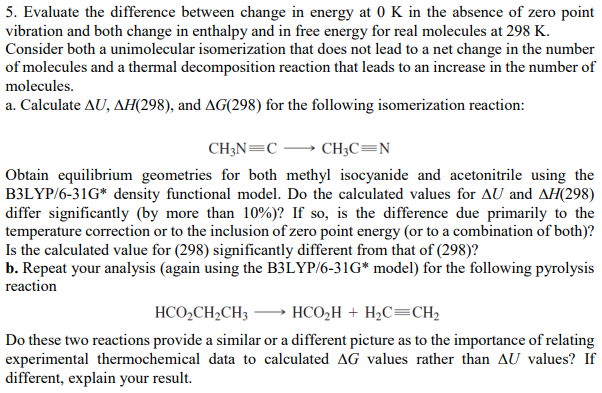
5. Evaluate the difference between change in energy at 0K in the absence of zero point vibration and both change in enthalpy and in free energy for real molecules at 298K. Consider both a unimolecular isomerization that does not lead to a net change in the number of molecules and a thermal decomposition reaction that leads to an increase in the number of molecules. a. Calculate U,H(298), and G(298) for the following isomerization reaction: CH3NCCH3CN Obtain equilibrium geometries for both methyl isocyanide and acetonitrile using the B3LYP/6-31G* density functional model. Do the calculated values for U and H(298) differ significantly (by more than 10% )? If so, is the difference due primarily to the temperature correction or to the inclusion of zero point energy (or to a combination of both)? Is the calculated value for (298) significantly different from that of (298)? b. Repeat your analysis (again using the B3LYP/6-31G* model) for the following pyrolysis reaction HCO2CH2CH3HCO2H+H2CCH2 Do these two reactions provide a similar or a different picture as to the importance of relating experimental thermochemical data to calculated G values rather than U values? If different, explain your result
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


