Question: 5. How does an adiabatic process compare to an isentropic process? (A) adiabatic: heat transfer =0; isentropic: heat transfer =0 (B) adiabatic: heat transfer =0;
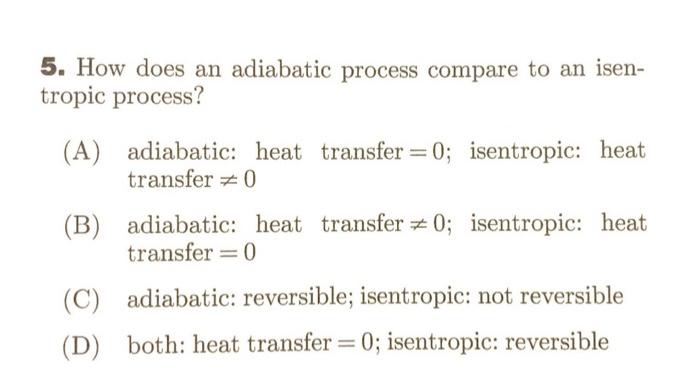
5. How does an adiabatic process compare to an isentropic process? (A) adiabatic: heat transfer =0; isentropic: heat transfer =0 (B) adiabatic: heat transfer =0; isentropic: heat transfer =0 (C) adiabatic: reversible; isentropic: not reversible (D) both: heat transfer =0; isentropic: reversible
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


