Question: 5. N2(8) 2NO(S) In which direction will the equilibrium shift...explain each..... + 0265) AH = +179 kJ a) addition of nitrogen gas b) heat the
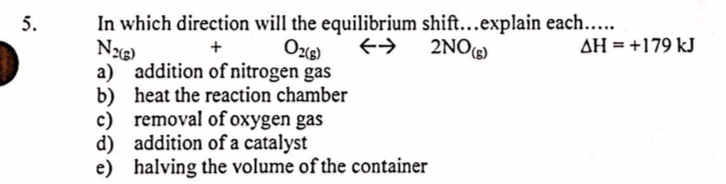
5. N2(8) 2NO(S) In which direction will the equilibrium shift...explain each..... + 0265) AH = +179 kJ a) addition of nitrogen gas b) heat the reaction chamber c) removal of oxygen gas d) addition of a catalyst e) halving the volume of the container
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


