Question: 5 points From the following equations and enthalpy changes , H ? f ( C O 2 g ) = - 3 9 3 .
points
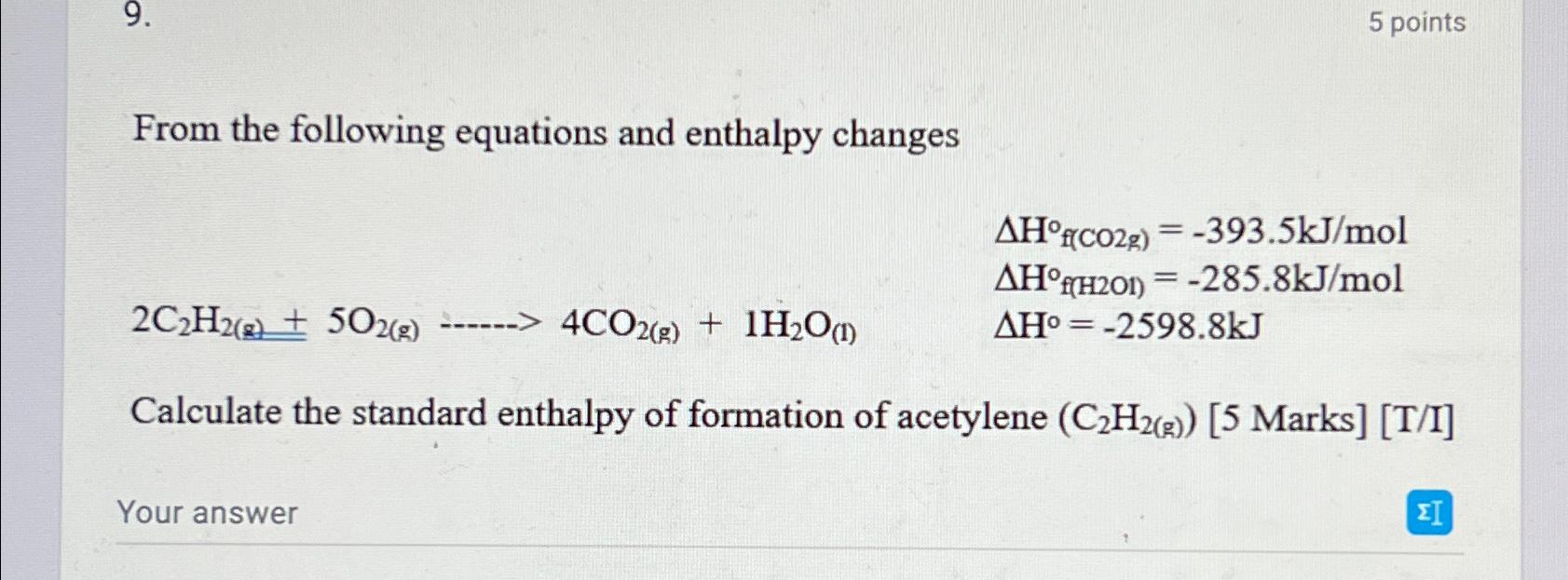
From the following equations and enthalpy changes
Calculate the standard enthalpy of formation of acetylene :Marks
Your answer
I
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


