Question: 5. Provide ALL reasonable resonance structures for each molecular structure below. Note that you need use the curved arrows to show how electrons are moved.
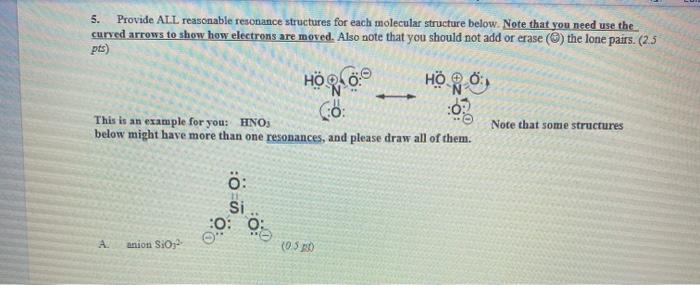


5. Provide ALL reasonable resonance structures for each molecular structure below. Note that you need use the curved arrows to show how electrons are moved. Also note that you should not add or erase () the lone pairs. (2.5 pts) HR This is an example for you: HNOS Co Note that some structures below might have more than one resonances, and please draw all of them. HORO: 0: Si 0.0. A anion SiO (03.10 : 0 B anion HSO? (0.5 p0) Ansuming that the phosphorus atom has the "superpower" of disobeying the Octet rule (It actually dose not, but we are just using this structure for practicing electron pushing, @). CH3 N CH3 . (0.25 Hint: Double check your resonance structure - does it have the same overall charge (negative one in this case)? Double check every answer of yours in problem 5. S: c-, 11 : D (0.23 20 Hint Most of time we can treat sulfur as oxygen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


