Question: 5) The equilibrium partial pressures for the reaction bellow are P(PCl3)=0.12atm, P(Cl2)=0.16atm and P(PCl5)=1.30atm at 455K. Find the value of the equilibrium constant, Kp. PCl3(g)+Cl2(g)PCl5(g)
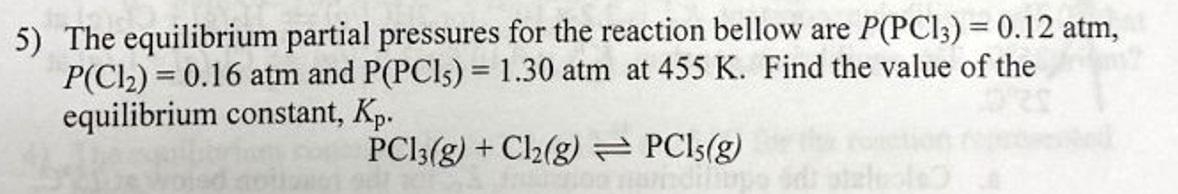
5) The equilibrium partial pressures for the reaction bellow are P(PCl3)=0.12atm, P(Cl2)=0.16atm and P(PCl5)=1.30atm at 455K. Find the value of the equilibrium constant, Kp. PCl3(g)+Cl2(g)PCl5(g)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


