Question: 5. The formula for a compound containing an unknown element, M, is M2(SO4)3. M is one of the following ions: Mg+2CircleMAl+3 C=95(F32) and K=C+273.151 pound
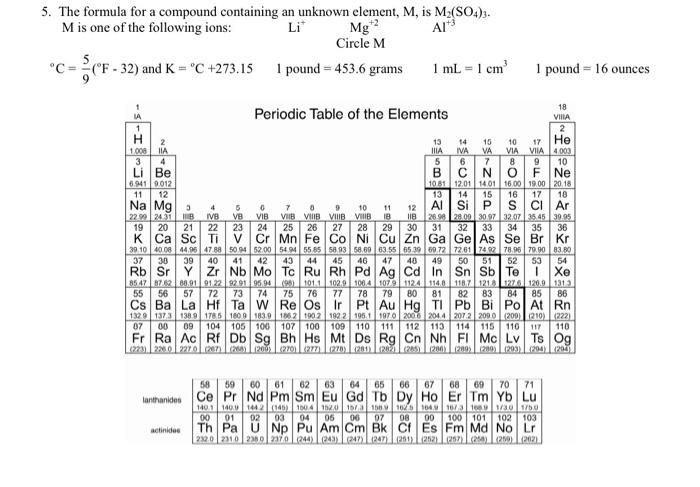
5. The formula for a compound containing an unknown element, M, is M2(SO4)3. M is one of the following ions: Mg+2CircleMAl+3 C=95(F32) and K=C+273.151 pound =453.6 grams 1mL=1cm31 pound =16 ounces 1A
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


