Question: 5. Use the Newton-Raphson method to calculate the molar volume of CO at T=300 K and P=10 atm from the Van der Waals equation
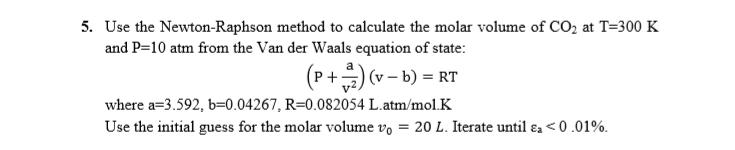
5. Use the Newton-Raphson method to calculate the molar volume of CO at T=300 K and P=10 atm from the Van der Waals equation of state: (P+) (v - b) = RT where a=3.592, b=0.04267, R=0.082054 L.atm/mol.K Use the initial guess for the molar volume vo = 20 L. Iterate until
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Oshiven Au P0 Vb RT Fiv P fx Here 9 3592 plo fv 10 3599 2 20 Pv So fv ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


