Question: 5.a) Circle the most electronegative atom and put a square around the least electronegative atom. (2 points) ClMgOCFBSFeAlHN b) Write the ground-state configuration of the
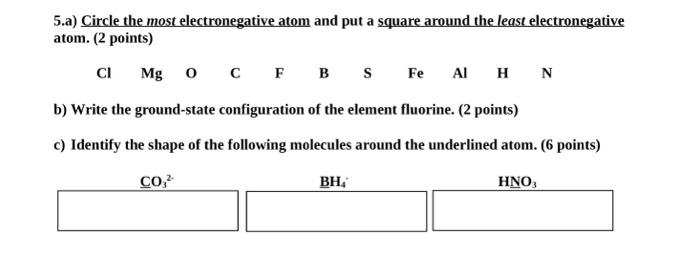
5.a) Circle the most electronegative atom and put a square around the least electronegative atom. (2 points) ClMgOCFBSFeAlHN b) Write the ground-state configuration of the element fluorine. ( 2 points) c) Identify the shape of the following molecules around the underlined atom. (6 points) CO32[BH4] HNO3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


