Question: 6. (10 pts) Bromothymol blue is a common acid base indicator which you may be familiar with. It is often used in situations where
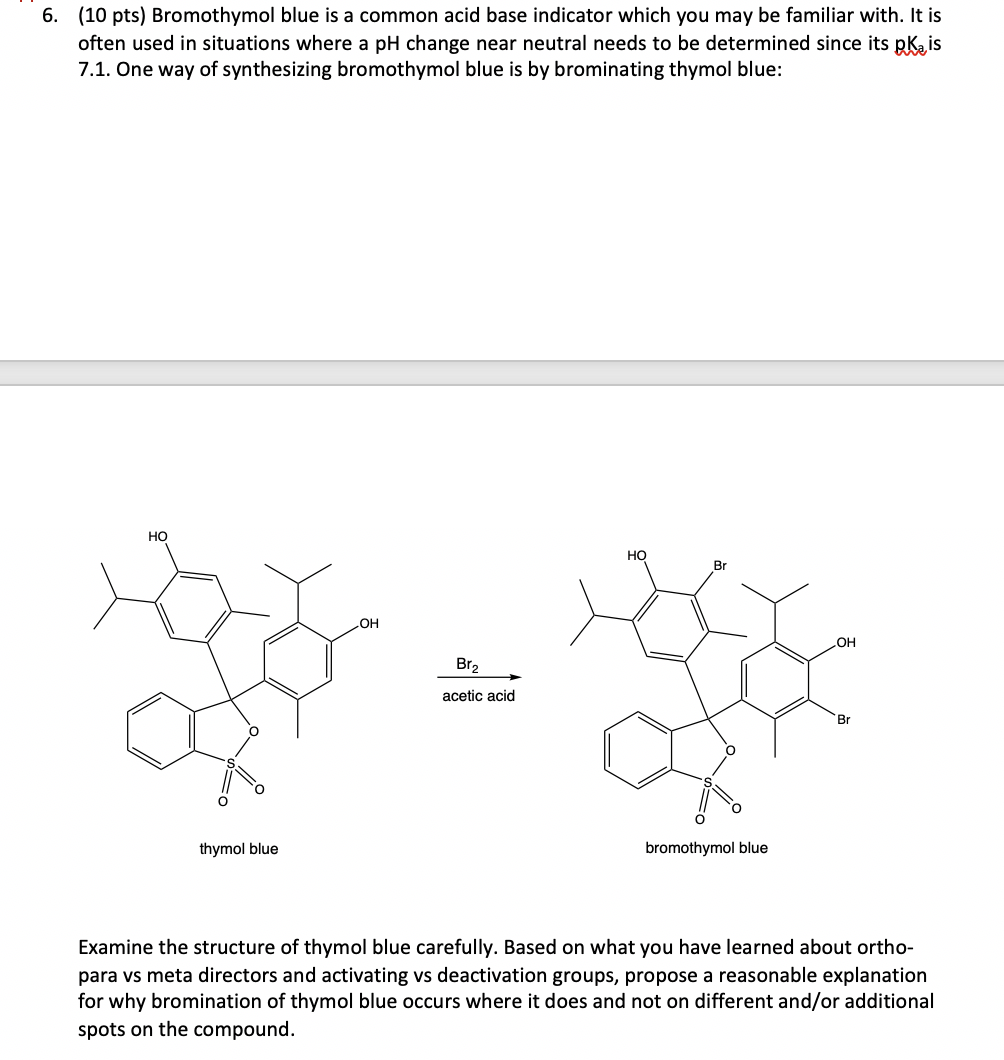
6. (10 pts) Bromothymol blue is a common acid base indicator which you may be familiar with. It is often used in situations where a pH change near neutral needs to be determined since its pkais 7.1. One way of synthesizing bromothymol blue is by brominating thymol blue: HO O thymol blue OH Br acetic acid HO Br O bromothymol blue OH Br Examine the structure of thymol blue carefully. Based on what you have learned about ortho- para vs meta directors and activating vs deactivation groups, propose a reasonable explanation for why bromination of thymol blue occurs where it does and not on different and/or additional spots on the compound.
Step by Step Solution
3.52 Rating (155 Votes )
There are 3 Steps involved in it
it is an As OH group has a love pais electron donating group It activates the ortho ... View full answer

Get step-by-step solutions from verified subject matter experts


