Question: 6. [-/16 Points] DETAILS A liquid in a pipe has a Reynolds number of 1700 with a flow rate of 0.00201 m /s. If the
![6. [-/16 Points] DETAILS A liquid in a pipe has a](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6c30275695_81066f6c3024b318.jpg)
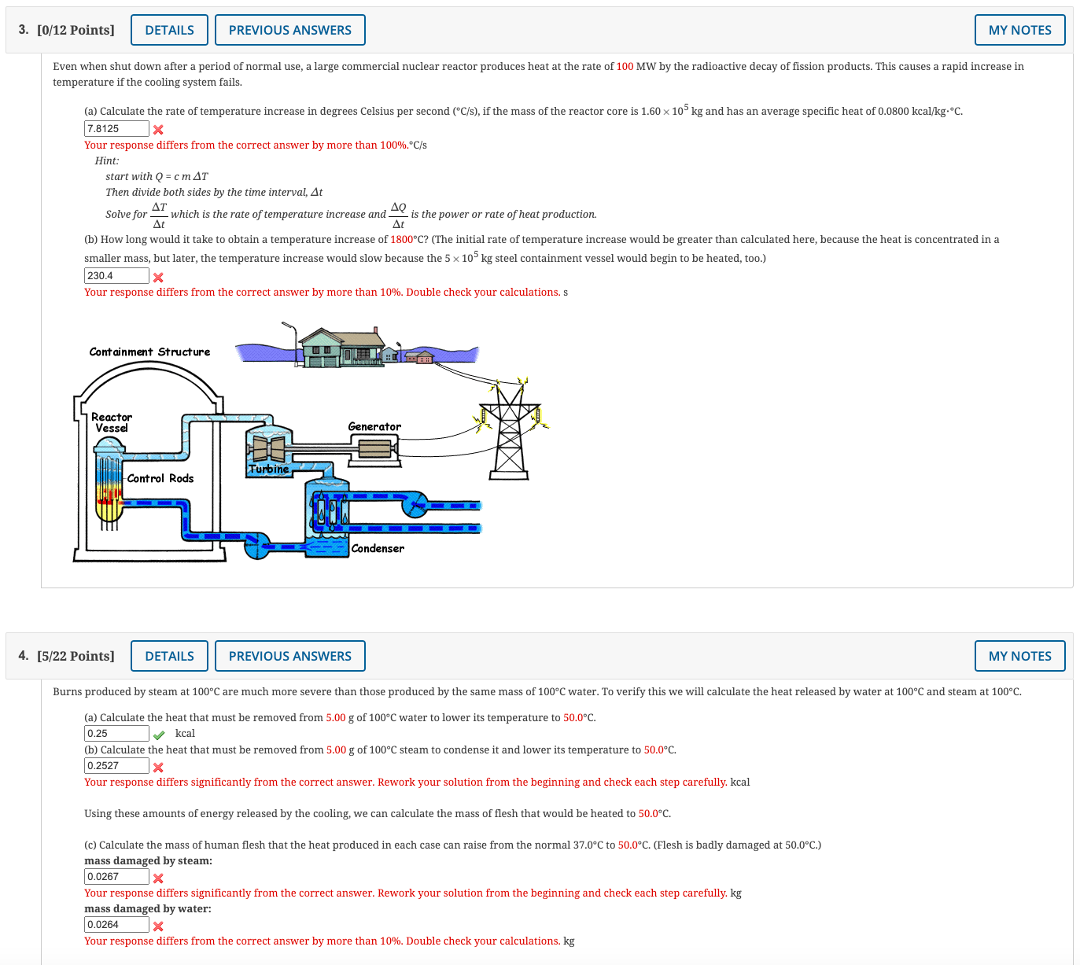

6. [-/16 Points] DETAILS A liquid in a pipe has a Reynolds number of 1700 with a flow rate of 0.00201 m /s. If the flow rate is increased by a factor of 2.1, what will the new Reynolds number be. Hint: Use the continuity equation, Q = v.A = V(AR?) = . V D2 -, to replace the speed in the usual formula for Reynolds number and obtain a version of the Reynolds number formula containing the flow rate. 4 If the flow rate increased by a factor of 2.1, the Reynolds number would become Assume that the viscosity, radius, and density did not change when the flow rate increased. Does the new Reynolds number indicate turbulent flow, laminar flow, or intermittent or unstable flow which could be either laminar or turbulent? The type of flow after the flow rate increase is expected to be O laminar O intermittent O turbulent Whether the flow is turbulent or laminar can depend on other properties such as the smoothness of the tubing, or how sharp the turns are in the tubing, or changes in the shape of the tubing. See the class notes for estimated values of the flow transitions.3. [0/12 Points] DETAILS PREVIOUS ANSWERS MY NOTES Even when shut down after a period of normal use, a large commercial nuclear reactor produces heat at the rate of 100 MW by the radioactive decay of fission products. This causes a rapid increase in temperature if the cooling system fails (a) Calculate the rate of temperature increase in degrees Celsius per second ("C/s), if the mass of the reactor core is 1.60 x 103 kg and has an average specific heat of 0.0800 kcal/kg-*C. 7.8125 X Your response differs from the correct answer by more than 100%. C/s Hint: start with Q = cmAT Then divide both sides by the time interval, At AL Solve for which is the rate of temperature increase and _ is the power or rate of heat production. (b) How long would it take to obtain a temperature increase of 1800*C? (The initial rate of temperature increase would be greater than calculated here, because the heat is concentrated in a smaller mass, but later, the temperature increase would slow because the 5 x 103 kg steel containment vessel would begin to be heated, too.) 230.4 X Your response differs from the correct answer by more than 10%. Double check your calculations. s Containment Structure Reactor Vessel Generator Turbine Control Rods Condenser 4. [5/22 Points] DETAILS PREVIOUS ANSWERS MY NOTES Burns produced by steam at 100"C are much more severe than those produced by the same mass of 100"C water. To verify this we will calculate the heat released by water at 100"C and steam at 100-C. (a) Calculate the heat that must be removed from 5.00 g of 100 C water to lower its temperature to 50.0 C. 0.25 kcal (b) Calculate the heat that must be removed from 5.00 g of 100"C steam to condense it and lower its temperature to 50.0*C. 0.2527 X Your response differs significantly from the correct answer. Rework your solution from the beginning and check each step carefully. kcal Using these amounts of energy released by the cooling, we can calculate the mass of flesh that would be heated to 50.0 C. (c) Calculate the mass of human flesh that the heat produced in each case can raise from the normal 37.0*C to 50.0*C. (Flesh is badly damaged at 50.0 C.) mass damaged by steam 0.0267 X Your response differs significantly from the correct answer. Rework your solution from the beginning and check each step carefully. kg mass damaged by water: 0.0264 Your response differs from the correct answer by more than 10%. Double check your calculations. kg11. [0/6 Points] DETAILS PREVIOUS ANSWERS MY NOTES A 0.0725 kg ice cube at -30.0*C is placed in 0.537 kg of 35.0"C water in a very well insulated container. What is the final temperature? The latent heat of fusion of water is 79.8 kcal/kg, the specific heat of ice is 0.50 kcal/(kg . "C), and the specific heat of water is 1.00 kcal/(kg . "C). 24.34 X Your response differs from the correct answer by more than 10%. Double check your calculations. How many different changes are involved for the ice to go from -30.0'C to melted water at some final temperature? How is the quantity of heat gained by the ice related to the quantity of heat lost by the water C5. [0/8 Points] DETAILS PREVIOUS ANSWERS MY NOTES The gauge pressure in your car tires is 2.00 x 10" N/mat a temperature of 35.0"C when you drive it onto a ferry boat to Alaska. What is their gauge pressure later, when their temperature has dropped to -40.0"C? Assume that their volume has not changed. 1.49 1x Your response differs from the correct answer by more than 10%. Double check your calculations. The ideal gas law relates volume, absolute temperature (K not C), and absolute pressure (not gauge pressure). atm (Use 1.013 x 103 Pa as 1 atm and -273.15*C as absolute zero temperature. Also assume that the air in the tire acts like an ideal gas. 1 N/m- = 1 Pa)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


