Question: 6. (3 + 3 = 6 points) Initial composition is 1 mol of CO2, 5 mol of H2, and 2 mol of CO. Develop equations
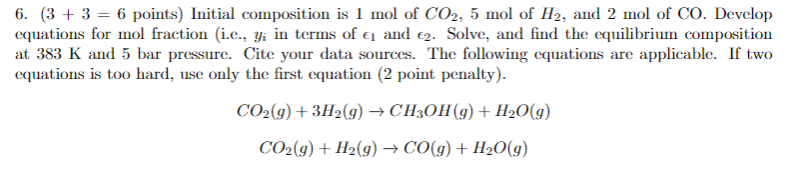
6. (3 + 3 = 6 points) Initial composition is 1 mol of CO2, 5 mol of H2, and 2 mol of CO. Develop equations for mol fraction i.e., yi in terms of & and 2. Solve, and find the equilibrium composition at 383 K and 5 bar pressure. Cite your data sources. The following equations are applicable. If two equations is too hard, use only the first equation (2 point penalty). CO2(g) + 3H2(g) + CH3OH(g) + H2O(g) CO2(g) + H2(g) CO(g) + H2O(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


