Question: 6 3 attempts left Check my work Enter your answer in the provided box. Hint 1 points Solution Sulfur dioxide is produced in enormous amounts
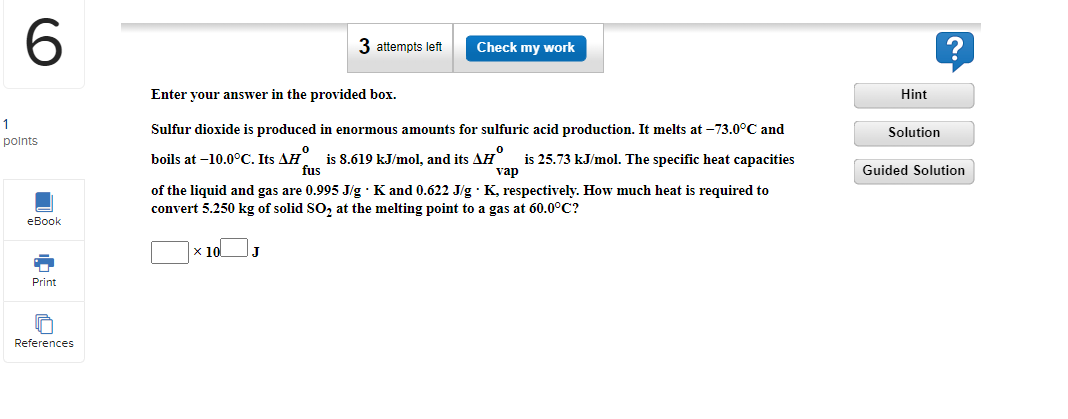
6 3 attempts left Check my work Enter your answer in the provided box. Hint 1 points Solution Sulfur dioxide is produced in enormous amounts for sulfuric acid production. It melts at -73.0C and boils at -10.0C. Its AH is 8.619 kJ/mol, and its AH is 25.73 kJ/mol. The specific heat capacities fus vap of the liquid and gas are 0.995 J/g. K and 0.622 Jg. K, respectively. How much heat is required to convert 5.250 kg of solid so, at the melting point to a gas at 60.0C? Guided Solution eBook x 10 Print References
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


