Question: 6. Decarburization is the decrease of the carbon content of (the surface of) a steel due to interactions with the environment at elevated temperatures.
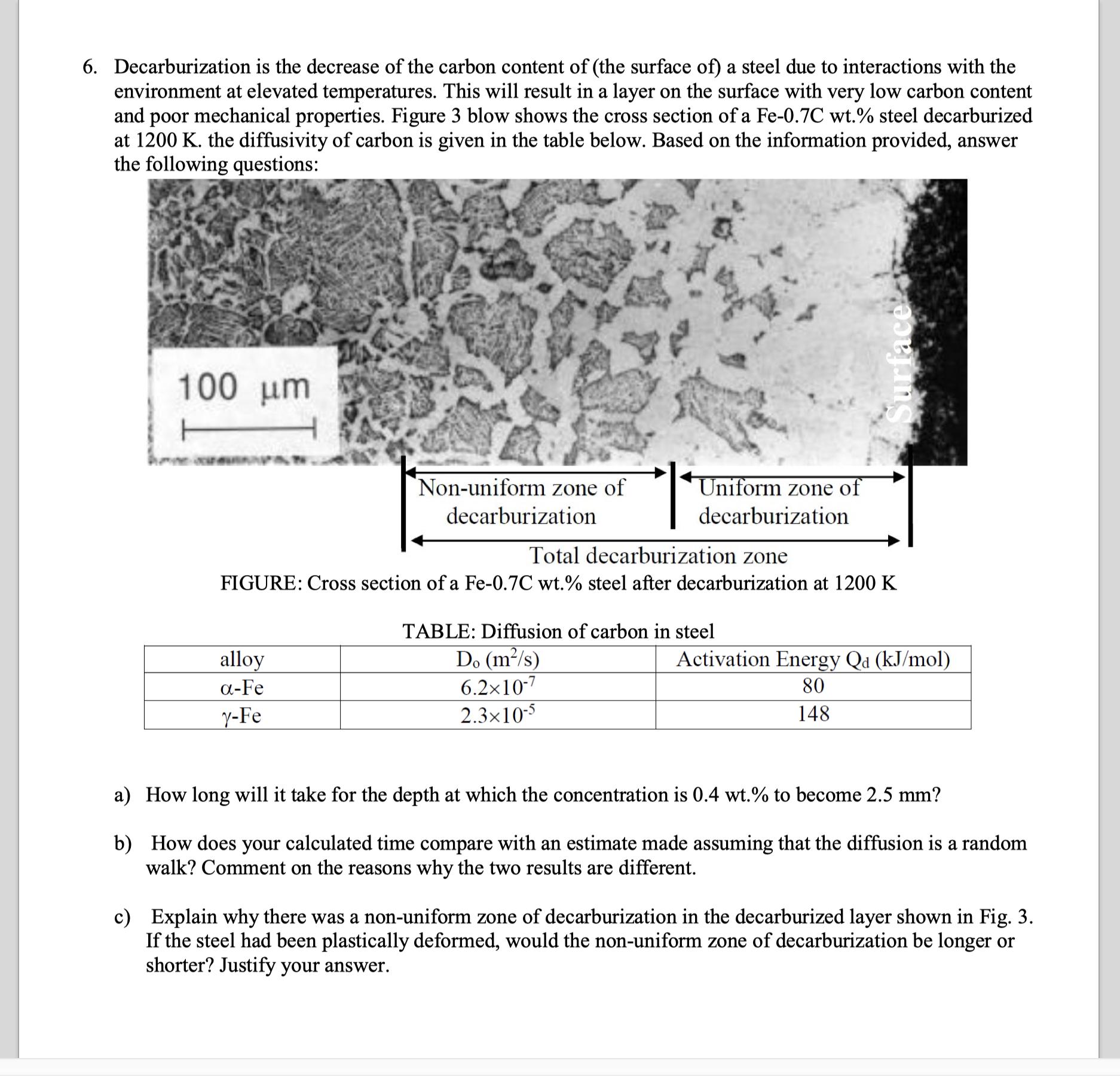
6. Decarburization is the decrease of the carbon content of (the surface of) a steel due to interactions with the environment at elevated temperatures. This will result in a layer on the surface with very low carbon content and poor mechanical properties. Figure 3 blow shows the cross section of a Fe-0.7C wt.% steel decarburized at 1200 K. the diffusivity of carbon is given in the table below. Based on the information provided, answer the following questions: 100 m Non-uniform zone of decarburization Uniform zone of decarburization Total decarburization zone FIGURE: Cross section of a Fe-0.7C wt.% steel after decarburization at 1200 K alloy -Fe y-Fe TABLE: Diffusion of carbon in steel Do (m/s) Activation Energy Qa (kJ/mol) 6.210-7 80 2.310-5 148 a) How long will it take for the depth at which the concentration is 0.4 wt.% to become 2.5 mm? b) How does your calculated time compare with an estimate made assuming that the diffusion is a random walk? Comment on the reasons why the two results are different. c) Explain why there was a non-uniform zone of decarburization in the decarburized layer shown in Fig. 3. If the steel had been plastically deformed, would the non-uniform zone of decarburization be longer or shorter? Justify your answer.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


