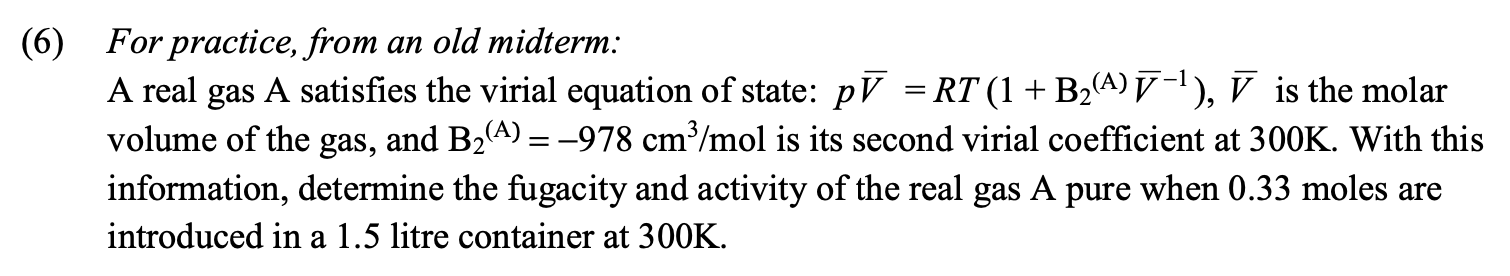
Question: ( 6 ) For practice, from an old midterm: A real gas A satisfies the virial equation of state: p b a r ( V
For practice, from an old midterm:
A real gas A satisfies the virial equation of state: is the molar
volume of the gas, and is its second virial coefficient at With this
information, determine the fugacity and activity of the real gas A pure when moles are
introduced in a litre container at
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


