Question: 6. For the given first order reaction 7. AB The half life of the reaction is 0.3010 min. The ration of the initial concentration

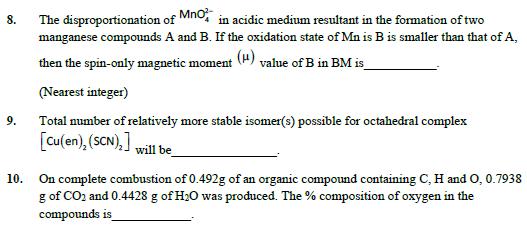
6. For the given first order reaction 7. AB The half life of the reaction is 0.3010 min. The ration of the initial concentration of reactant to the concentration of reactant at time 2.0 min will be equal to (Nearest integer) The number of interhalogens from the following having square pyramidal structure is: CIF3, IF7, BrF5, BrF3, I2C16, IFS, CIF, CIF5 8. 9. The disproportionation of MnO in acidic medium resultant in the formation of two manganese compounds A and B. If the oxidation state of Mn is B is smaller than that of A, then the spin-only magnetic moment (H) value of B in BM is (Nearest integer) Total number of relatively more stable isomer(s) possible for octahedral complex [Cu(en), (SCN),] will be 10. On complete combustion of 0.492g of an organic compound containing C, H and O, 0.7938 g of CO2 and 0.4428 g of H2O was produced. The % composition of oxygen in the compounds is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


