Question: 6 . In free - radical polymerization, ( a ) Calculate a number - average molar mass of polystyrene prepared by bulk radical polymerization. (
In freeradical polymerization,
a Calculate a numberaverage molar mass of polystyrene prepared by bulk radical polymerization.
b If the light lamp is suddenly broken in the middle of initiation stage, explain briefly possible variations in the
rate of polymerization and degree of polymerization. No need to use equations.
Assumptions: The initial concentration of the benzophenone as an initiator is ; Quantum
yield of benzophenone is ; Molar absorptivity of the initiator is ;
Intensity of incident light is ; The rate coefficients for propagation and termination
are and respectively; The density of styrene at the polymerization
temperature is ; Termination by disproportionation is negligible; Chain transfer is
negligible; Neglect contributions from end groups; The ultraviolet light lamp in
wavelength is used in this experiment; Steady state condition.
Hint:
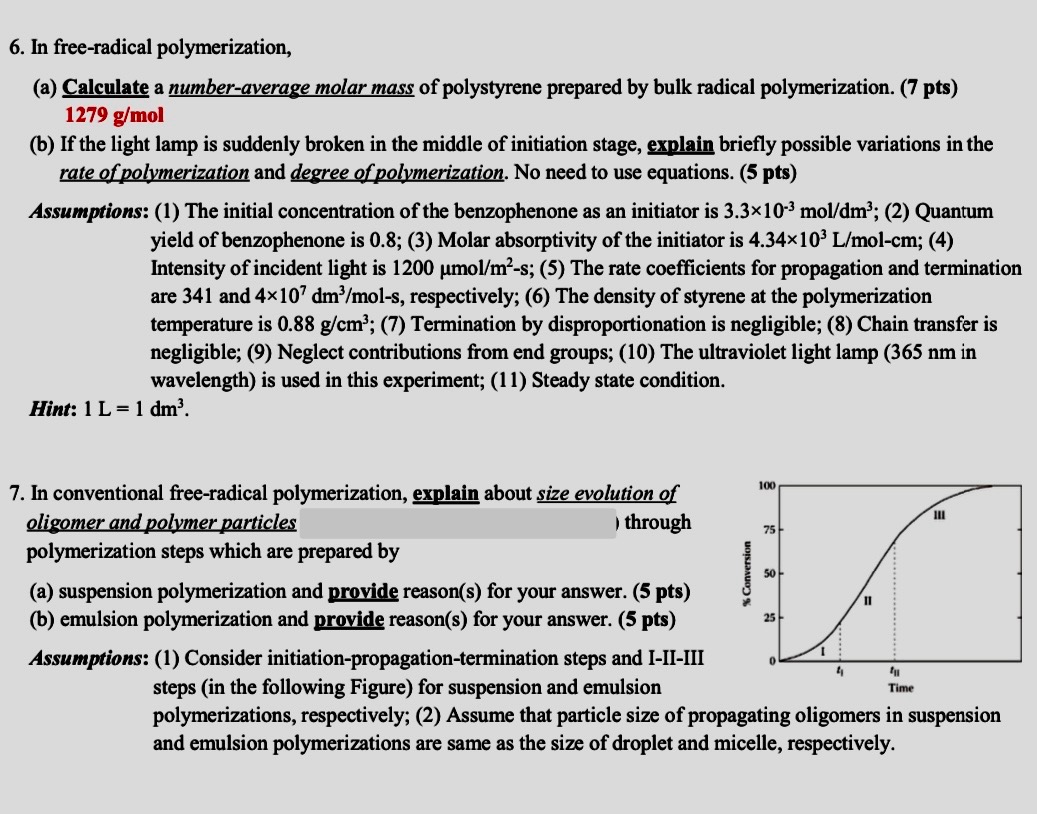
In conventional freeradical polymerization, explain about size evolution of
oligomer and polymer particles
through
polymerization steps which are prepared by
a suspension polymerization and provide reasons for your answer.
b emulsion polymerization and provide reasons for your answer.
Assumptions: Consider initiationpropagationtermination steps and IIIIII
steps in the following Figure for suspension and emulsion
polymerizations, respectively; Assume that particle size of propagating oligomers in suspension
and emulsion polymerizations are same as the size of droplet and micelle, respectively.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


