Question: 6. Study the pV graph below for an ideal gas containing 3.3 moles. Assume that the units for the graph axes are both standard international
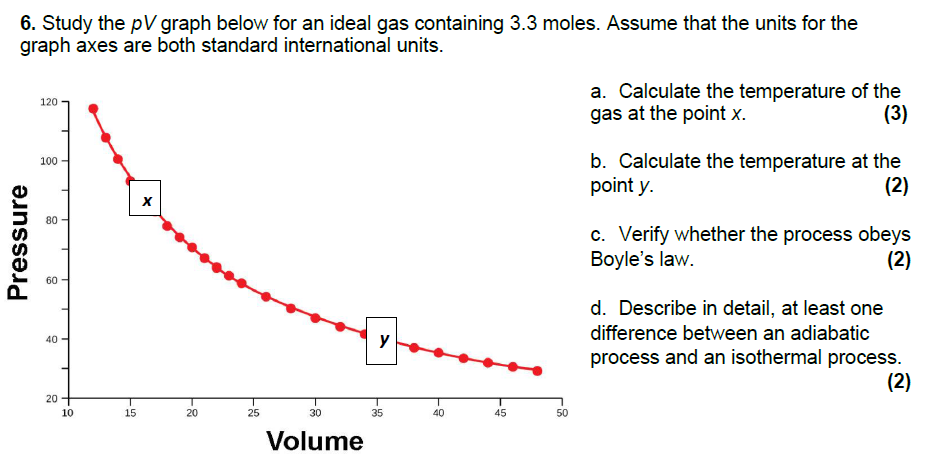
6. Study the pV graph below for an ideal gas containing 3.3 moles. Assume that the units for the graph axes are both standard international units. 120 a. Calculate the temperature of the gas at the point x. (3) 100 b. Calculate the temperature at the point y. (2) 80 Pressure c. Verify whether the process obeys Boyle's law. (2) 60 - 40 T d. Describe in detail, at least one difference between an adiabatic process and an isothermal process. (2) L 1 T E 20 10 15 20 25 30 35 40 45 50 Volume
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


