Question: 6. Use the thermochemical data about the element Silver (Ag) below: C mp Ly bp 2.4 x 102 J/kg 'C 1.1 x 105 J/kg 962
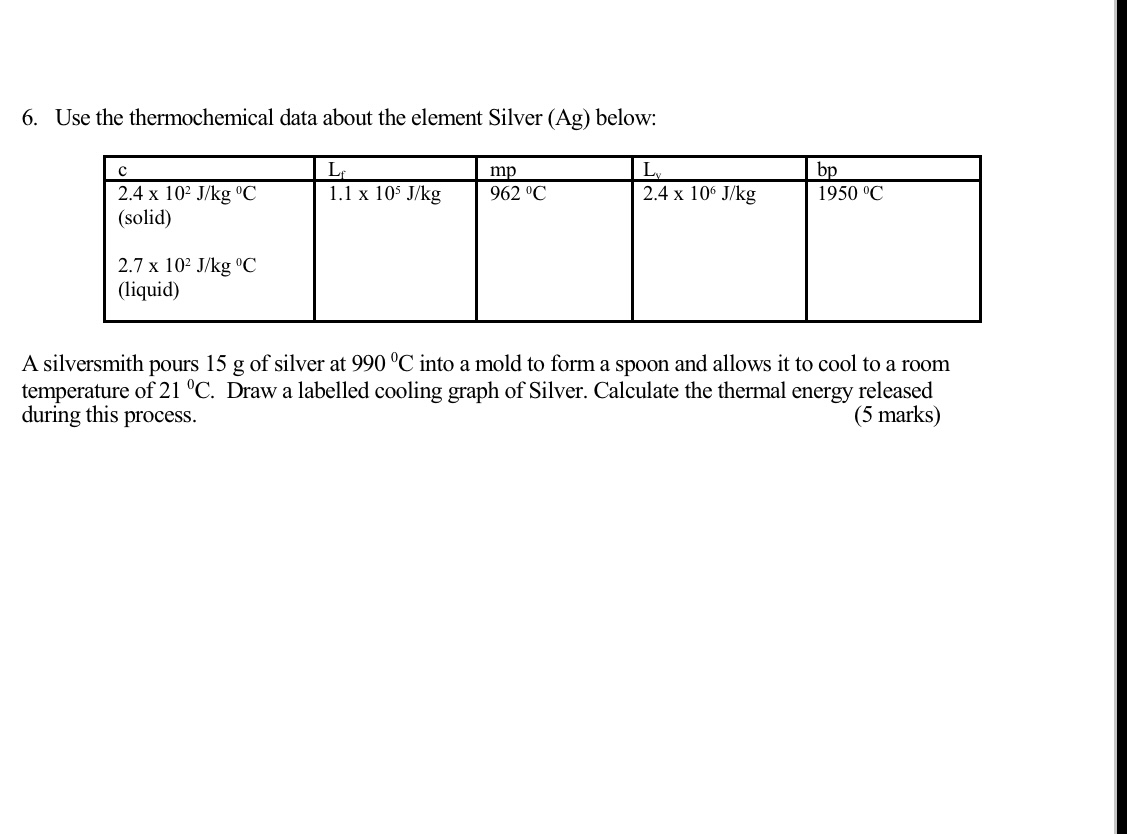
6. Use the thermochemical data about the element Silver (Ag) below: C mp Ly bp 2.4 x 102 J/kg 'C 1.1 x 105 J/kg 962 C 2.4 x 106 J/kg 1950 C (solid) 2.7 x 102 J/kg "C (liquid) A silversmith pours 15 g of silver at 990 "C into a mold to form a spoon and allows it to cool to a room temperature of 21 C. Draw a labelled cooling graph of Silver. Calculate the thermal energy released during this process
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


