The heat of combustion of butane is 22877 kJ/mol. Use this value to find the heat of
Question:
The heat of combustion of butane is 22877 kJ/mol. Use this value to find the heat of formation of butane. (You may also need to use additional thermochemical data found in Appendix E.)
Data from appendix E
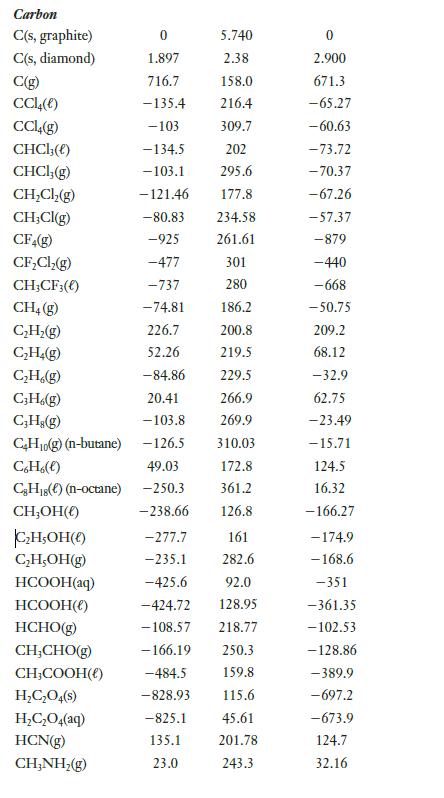
Transcribed Image Text:
Carbon C(s, graphite) C(s, diamond) C(g) CC14(e) CCL4(g) CHCI;(() CHCl, (g) CH₂Cl₂(g) CH₂Cl(g) CF4(g) CF₂Cl₂(g) CH3CF3(0) CH₂(g) C₂H₂(g) C₂H₂(g) C₂H.(g) C3H6(g) C₂H₂(g) C₂H₁0(g) (n-butane) C6H6(e) CH₁8(e) (n-octane) CH₂OH() C₂H5OH() C₂H,OH(g) HCOOH(aq) HCOOH() HCHO(g) CH,CHO(g) CH₂COOH() H₂C₂O4(s) H₂C₂O4(aq) HCN(g) CH,NH,(g) 0 1.897 716.7 -135.4 -103 -134.5 -103.1 - 121.46 -80.83 -925 -477 -737 -74.81 226.7 52.26 5.740 2.38 158.0 216.4 -277.7 -235.1 -425.6 -424.72 -108.57 309.7 202 295.6 177.8 234.58 261.61 301 280 186.2 200.8 219.5 -84.86 229.5 20.41 266.9 -103.8 269.9 -126.5 310.03 49.03 172.8 -250.3 361.2 -238.66 126.8 161 282.6 92.0 128.95 218.77 -166.19 250.3 -484.5 159.8 -828.93. 115.6 -825.1 45.61 135.1 201.78 23.0 243.3 0 2.900 671.3 -65.27 -60.63 -73.72 -70.37 -67.26 -57.37 -879 -440 -668 -50.75 209.2 68.12 -32.9 62.75 -23.49 -15.71 124.5 16.32 -166.27 -174.9 - 168.6 -351 -361.35 -102.53 -128.86 -389.9 -697.2 -673.9 124.7 32.16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)

Answered By

Sandip Nandnawar
I am a B.E (Information technology) from GECA and also have an M.C.M from The University of RTMNU, MH.
I worked as a software developer (Programmer and TL). Also working as an expert for the last 6 years and deal with complex assessment and projects. I have a team and lead a team of experts and conducted primary and secondary research. I am a senior software engg and senior expert and deal with all types of CSE and IT and other IT-related assessments and projects and homework.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The heat of combustion of decahydronaphthalene (C10H18) is -6286 kJ / mol The heat of combustion of naphthalene (C10H8) is-5157 kJ / mol. [In both cases CO2(g) and H2O(l) are the products.] Using...
-
Describe how a promotion discount can communicate information about a product.
-
Nisha has completed her MBA and has joined a company which was going to raise fund from long term sources such as Debt and Equity. Nisha was asked by her manager to prepare a report on which could be...
-
Slapshot Company makes ice hockey sticks. Last week, direct materials (wood, paint, Kevlar, and resin) costing $24,000 were put into production. Direct labor of $40,000 (20 workers x 100 hours x $20...
-
The sales projection for your retail business is $650,000. The industry average for the asset turnover ratio is 5. How much inventory (total assets) should you plan to stock?
-
On March 13, 2009, Juan Mendez Sr. was admitted to a nursing facility. On that day, a doctor employed by the facility determined the father lacked the capacity to give informed consent or make...
-
TechSystem manufactures an optical switch that it uses in its final product. TechSystems incurred the following manufacturing costs when it produced 68,000 units last year: TechSystems does not yet...
-
c) Show that 2x+1 x (x+1) 1-2x2 = 2x + Hence, evaluate xx+1) S 2x+1 dx by first expressing the integrand as sum of partial fractions.
-
Hydrogen gas will react with either acetylene or ethylene gas. The thermochemical equations for these reactions are provided below. Write the thermochemical equation for the conversion of acetylene...
-
The phase change between graphite and diamond is difficult to observe directly. Both substances can be burned, however. From these equations, calculate H for the conversion of diamond into graphite....
-
Define HR audit. Bring out its scope and approaches.
-
M Inc., a Canadian public corporation, carries on business across Canada. In the current year M Inc. has taxable income of $500,000. What is the amount of federal tax payable?
-
Mr. Z split his retirement fund of $268,000 between a stock index ETF and a long-term bond ETF in January 2004. At the anniversary, he reviewed the performance of these fund and found that the...
-
Find a "clickwrap" contract you have entered into, as defined in the course text. Make a copy, read it carefully, and attach it to your submitted assignment. Next, answer these questions: What...
-
The director wanted to review the financial reports of its company. Thus, requested it from the accountant. However, the accountant refused to issue the reports to him. Identify and explain which...
-
NUMBER ONE a) b) Differentiate between a feedback control system and a feed forward control system. (4 marks) In his study of: "the impact of budgets on people" C Argyris reported the following...
-
The following is a news item reported by Reuters: WASHINGTON, Jan 29 (Reuters)Wright Medical Group, a maker of reconstructive implants for knees and hips, on Tuesday filed to sell 3 million shares of...
-
Identify the most stable compound:
-
Two operators can be applied to a function in succession. By definition, AB f(x) = A [B f(x)]. Evaluate AB f(x) if A = d /dx, B = x, and f (x) = cos x.
-
Cos x an eigenfunction of the operator A if A f (x) = xf (x)?
-
Identify the reagents that you would use to accomplish each of the following transformations: (a) (b) H.
-
Give me the references for these points: Time Convenience: Time convenience refers to the ability of hotels to offer time-saving services and minimize guest effort. In Selangor, hotels can provide...
-
The Milwaukee plant of Healthy Life Styles, Inc. produces low-fat salad dressing. The following data pertain to the year just ended. Work in process, January 1 Units 30,000 gal. Percentage of...
-
Sharifi Hospital bases its budgets on patient-visits. The hospital's static budget for October appears below: Budgeted number of patient-visits Budgeted variable overhead costs: Supplies (@$5.20 per...

Study smarter with the SolutionInn App


