Question: 6) Using the composition given below, calculate the density (at 4000 Psi and 160 F) of the gas phase and liquid phase by using the
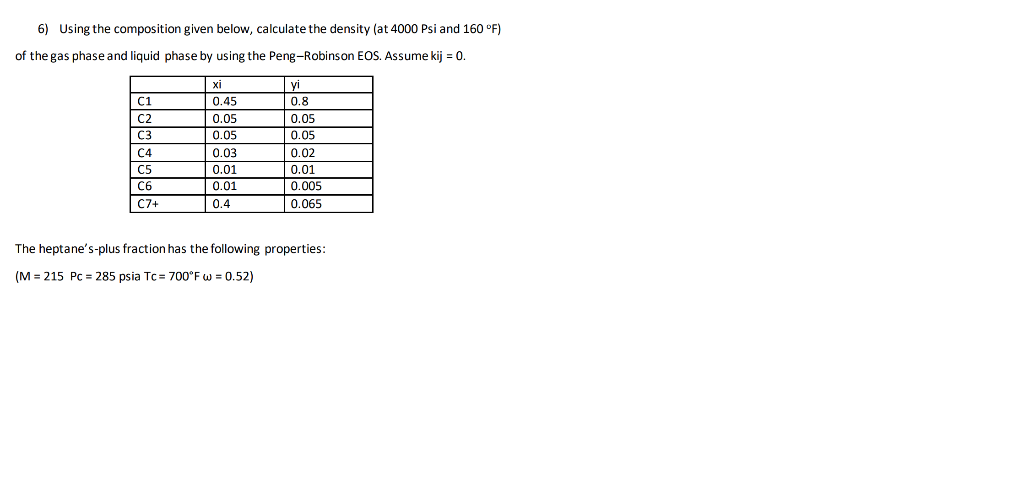
6) Using the composition given below, calculate the density (at 4000 Psi and 160 F) of the gas phase and liquid phase by using the Peng-Robinson EOS. Assume kij = 0. C1 C2 C3 C4 C5 C6 C7+ xi 0.45 0.05 0.05 0.03 0.01 0.01 0.4 yi 0.8 0.05 0.05 0.02 0.01 0.005 0.065 ee) The heptane's-plus fraction has the following properties: (M = 215 Pc = 285 psia Tc= 700F W = 0.52)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


