Question: 6.16. Using a color indicator which shows when the concentration of A falls below 0.1mol/ liter, the following scheme is devised to explore the kinetics
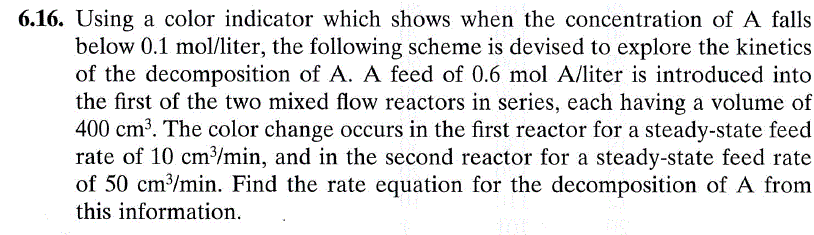
6.16. Using a color indicator which shows when the concentration of A falls below 0.1mol/ liter, the following scheme is devised to explore the kinetics of the decomposition of A. A feed of 0.6molA/ liter is introduced into the first of the two mixed flow reactors in series, each having a volume of 400cm3. The color change occurs in the first reactor for a steady-state feed rate of 10cm3/min, and in the second reactor for a steady-state feed rate of 50cm3/min. Find the rate equation for the decomposition of A from this information
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


