Question: ****6.2.13. The flowsheet shown in Figure P6.2.13 represents the process for the production of titanium dioxide (TiO2) used by Canadian Titanium Pigments at Varennes, Quebec.
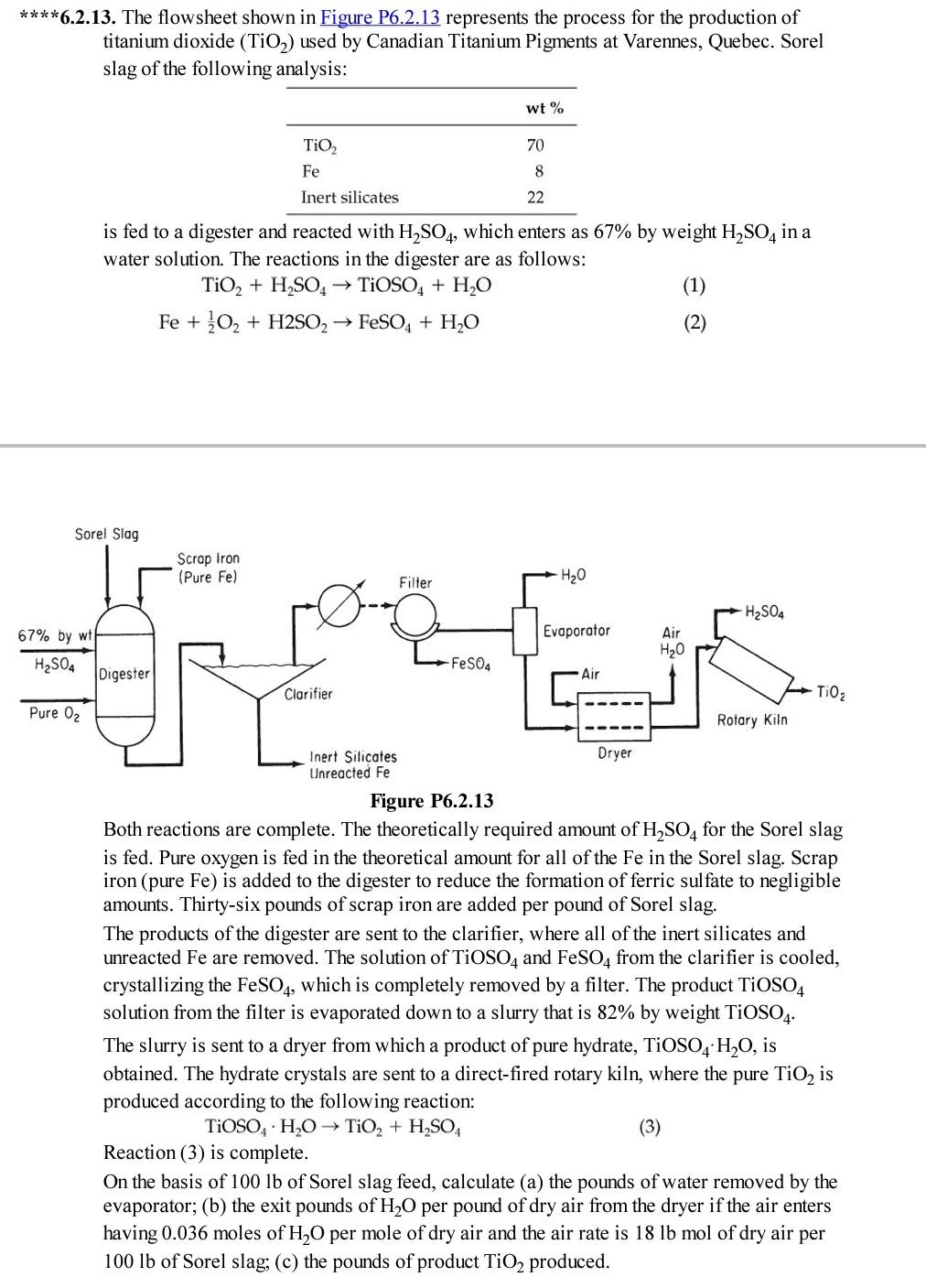
****6.2.13. The flowsheet shown in Figure P6.2.13 represents the process for the production of titanium dioxide (TiO2) used by Canadian Titanium Pigments at Varennes, Quebec. Sorel slag of the following analysis: wt% 70 TiO2 Fe 8 Inert silicates 22 is fed to a digester and reacted with H2SO4, which enters as 67% by weight H2SO4 in a water solution. The reactions in the digester are as follows: TiO2 + H2SO4 TIOSO4 + H2O (1) Fe + O2 + H2SO2 FeSO4 + H2O EE Sorel Slag Scrap Iron (Pure Fe) Filter HO H2SO4 67% by wt Evaporator Air H2O H2SO4 -FeSO4 Digester -Air Clarifier Tiog Pure 02 Rotary Kiln Inert Silicates Dryer Unreacted Fe Figure P6.2.13 Both reactions are complete. The theoretically required amount of H2SO4 for the Sorel slag is fed. Pure oxygen is fed in the theoretical amount for all of the Fe in the Sorel slag. Scrap iron (pure Fe) is added to the digester to reduce the formation of ferric sulfate to negligible amounts. Thirty-six pounds of scrap iron are added per pound of Sorel slag. The products of the digester are sent to the clarifier, where all of the inert silicates and unreacted Fe are removed. The solution of TiOSO4 and FeSO4 from the clarifier is cooled, crystallizing the FeSO4, which is completely removed by a filter. The product TiOSO4 solution from the filter is evaporated down to a slurry that is 82% by weight TiOS04. The slurry is sent to a dryer from which a product of pure hydrate, TiOSO4 H20, is a obtained. The hydrate crystals are sent to a direct-fired rotary kiln, where the pure TiO2 is produced according to the following reaction: TiOSO, H2O TiO2 + H2SO4 (3) Reaction (3) is complete. On the basis of 100 lb of Sorel slag feed, calculate (a) the pounds of water removed by the evaporator; (b) the exit pounds of H20 per pound of dry air from the dryer if the air enters having 0.036 moles of H20 per mole of dry air and the air rate is 18 lb mol of dry air per 100 lb of Sorel slag; (c) the pounds of product TiO2 produced
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


