Question: JUST DRAW THE CORRECT FLOWSHEET Shown below is a process for producing concentrated hydrogen cyanide, H C N , by the following overall reaction catalyzed
JUST DRAW THE CORRECT FLOWSHEET
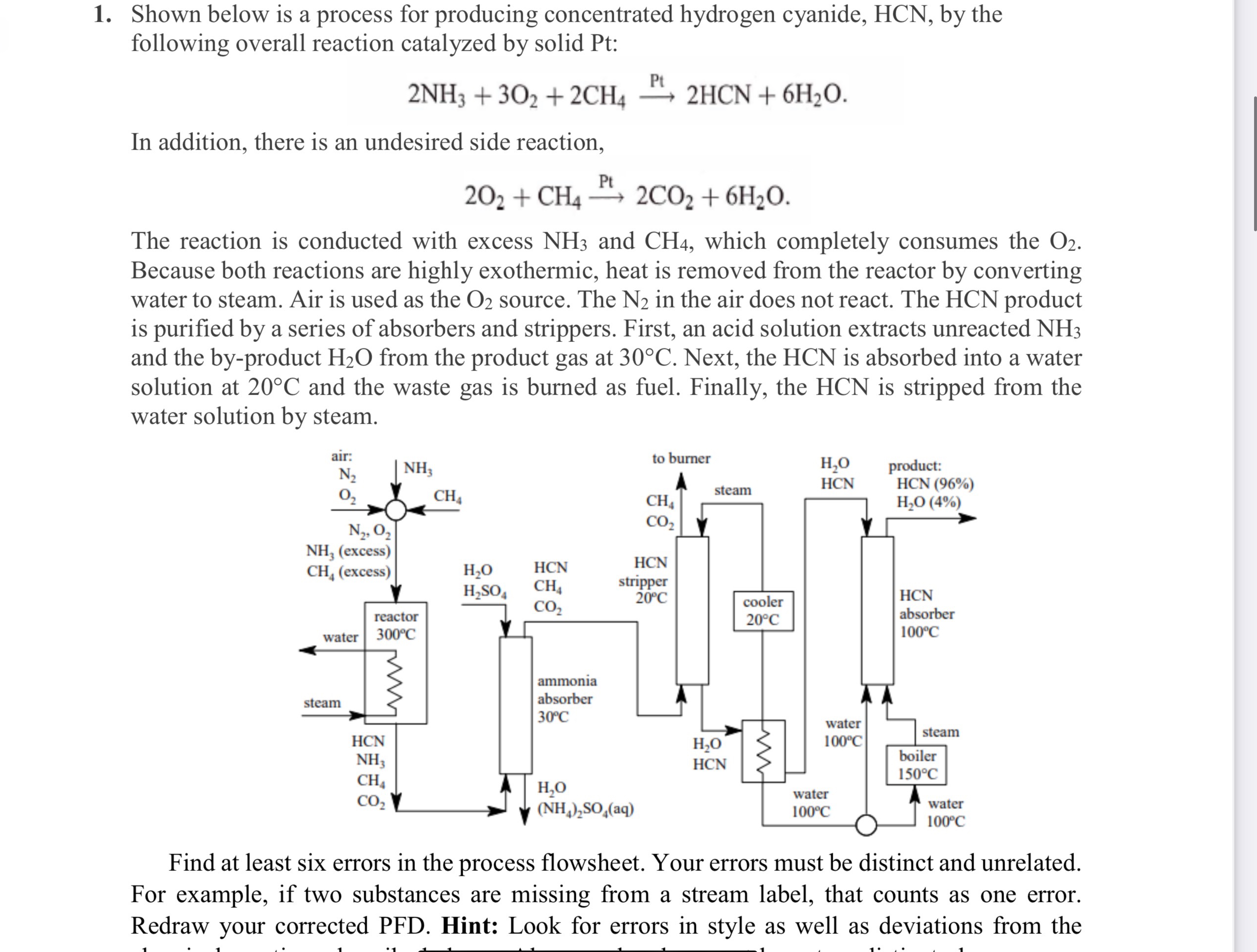
Shown below is a process for producing concentrated hydrogen cyanide, by the following overall reaction catalyzed by solid Pt:
In addition, there is an undesired side reaction,
The reaction is conducted with excess and which completely consumes the Because both reactions are highly exothermic, heat is removed from the reactor by converting water to steam. Air is used as the source. The in the air does not react. The product is purified by a series of absorbers and strippers. First, an acid solution extracts unreacted and the byproduct from the product gas at Next, the is absorbed into a water solution at and the waste gas is burned as fuel. Finally, the is stripped from the water solution by steam.
Find at least six errors in the process flowsheet. Your errors must be distinct and unrelated. For example, if two substances are missing from a stream label, that counts as one error. Redraw your corrected PFD Hint: Look for errors in style as well as deviations from the
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


